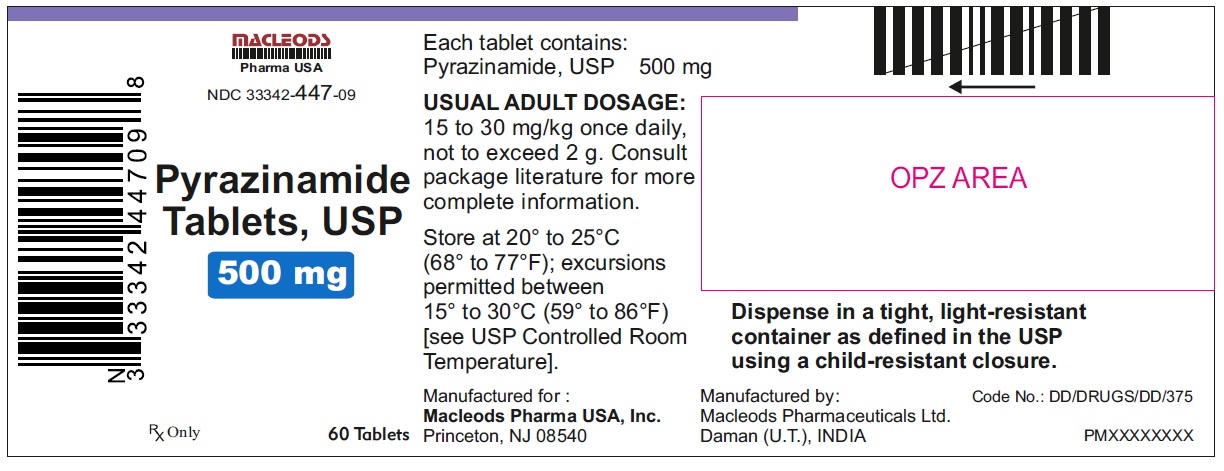
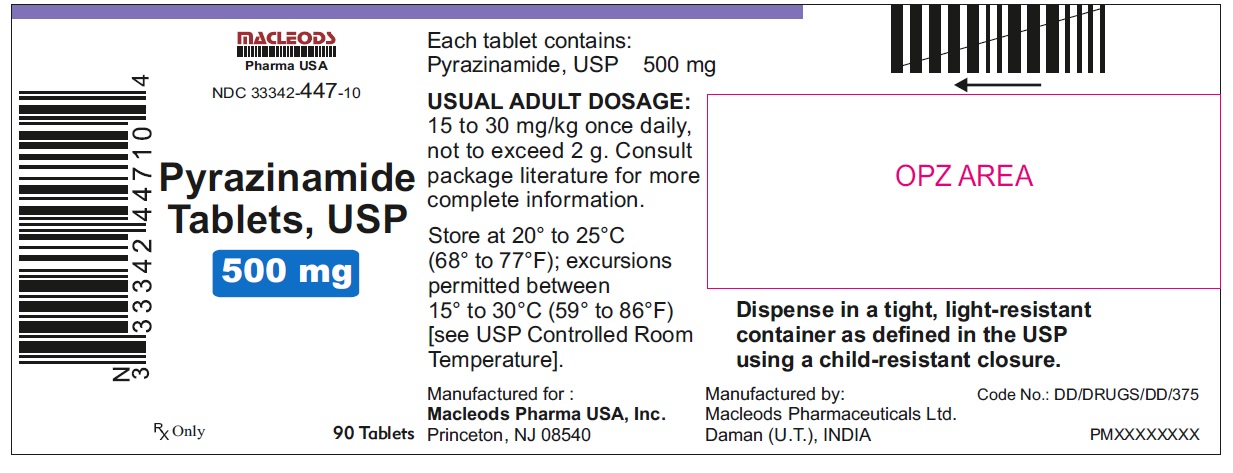
Label: PYRAZINAMIDE tablet
-
NDC Code(s):
33342-447-03,
33342-447-07,
33342-447-09,
33342-447-10, view more33342-447-11, 33342-447-12, 33342-447-15
- Packager: Macleods Pharmaceuticals Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 6, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION:
Pyrazinamide, USP the pyrazine analogue of nicotinamide, is an antituberculous agent. It is a white crystalline powder, stable at room temperature, and sparingly soluble in water. Pyrazinamide USP has the following structural formula:
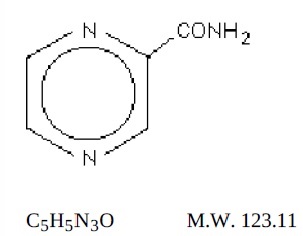
Each pyrazinamide tablet, USP for oral administration contains 500 mg of pyrazinamide and the following inactive ingredients: corn starch, colloidal silicon dioxide, magnesium stearate, povidone, sodium starch glycolate and talc.
-
CLINICAL PHARMACOLOGY:
Pyrazinamide is well absorbed from the GI tract and attains peak plasma concentrations within 2 hours.
Plasma concentrations generally range from 30 to 50 mcg/mL with doses of 20 to 25 mg/kg. It is widely distributed in body tissues and fluids including the liver, lungs and cerebrospinal fluid (CSF). The CSF concentration is approximately equal to concurrent steady-state plasma concentrations in patients with inflamed meninges.1 Pyrazinamide is approximately 10% bound to plasma proteins.2The half-life (t1/2) of pyrazinamide is 9 to 10 hours in patients with normal renal and hepatic function. The plasma half-life may be prolonged in patients with impaired renal or hepatic function. Pyrazinamide is hydrolyzed in the liver to its major active metabolite, pyrazinoic acid. Pyrazinoic acid is hydroxylated to the main excretory product, 5-hydroxypyrazinoic acid.3
Approximately 70% of an oral dose is excreted in the urine, mainly by glomerular filtration within 24 hours.3
Pyrazinamide may be bacteriostatic or bactericidal against Mycobacterium tuberculosis depending on the concentration of the drug attained at the site of infection. The mechanism of action is unknown. In vitro and in vivo the drug is active only at a slightly acidic pH.
Susceptibility Testing
For specific information regarding susceptibility test interpretive criteria and associated test methods and quality control standards recognized by FDA for this drug, please see: https://www.fda.gov/STIC. -
INDICATIONS & USAGE:
Pyrazinamide is indicated for the initial treatment of active tuberculosis in adults and children when combined with other antituberculous agents. (The current recommendation of the CDC for drug- susceptible disease is to use a six-month regimen for initial treatment of active tuberculosis, consisting of isoniazid, rifampin and pyrazinamide given for 2 months, followed by isoniazid and rifampin for 4 months.* 4 )
(Patients with drug-resistant disease should be treated with regimens individualized to their situation. Pyrazinamide frequently will be an important component of such therapy.)
(In patients with concomitant HIV infection, the physician should be aware of current recommendation of CDC. It is possible these patients may require a longer course of treatment).
It is also indicated after treatment failure with other primary drugs in any form of active tuberculosis.
Pyrazinamide should only be used in conjunction with other effective antituberculous agents.
*See recommendations of Center for Disease Control (CDC) and American Thoracic Society for complete regimen and dosage recommendations.4
- CONTRAINDICATIONS:
-
WARNINGS:
Patients started on pyrazinamide should have baseline serum uric acid and liver function determinations.
Those patients with preexisting liver disease or those at increased risk for drug related hepatitis (e.g.,alcohol abusers) should be followed closely.Pyrazinamide should be discontinued and not be resumed if signs of hepatocellular damage or hyperuricemia accompanied by an acute gouty arthritis appear.
-
PRECAUTIONS:
General:
Pyrazinamide inhibits renal excretion of urates, frequently resulting in hyperuricemia which is usually asymptomatic. If hyperuricemia is accompanied by acute gouty arthritis, pyrazinamide should be discontinued.
Pyrazinamide should be used with caution in patients with a history of diabetes mellitus, as management may be more difficult.
Primary resistance of M. tuberculosis to pyrazinamide is uncommon. In cases with known or suspected drug resistance, in vitro susceptibility tests with recent cultures of M. tuberculosis against pyrazinamide and the usual primary drugs should be performed. There are few reliable in vitro tests for pyrazinamide resistance. A reference laboratory capable of performing these studies must be employed.
Information for Patients:
Patients should be instructed to notify their physicians promptly if they experience any of the following: fever, loss of appetite, malaise, nausea and vomiting, darkened urine, yellowish discoloration of the skin and eyes, pain or swelling of the joints.
Compliance with the full course of therapy must be emphasized, and the importance of not missing any doses must be stressed.
Laboratory Tests:
Baseline liver function studies [especially ALT (SGPT), AST (SGOT) determinations] and uric acid levels should be determined prior to therapy. Appropriate laboratory testing should be performed at periodic intervals and if any clinical signs of symptoms occur during therapy.
Drug/Laboratory Test Interactions:
Pyrazinamide has been reported to interfere with ACETEST® and KETOSTIX® urine tests to produce a pink-brown color. 5
Carcinogenicity , Mutagenicity, Impairment of Fertility:6,7,8
In lifetime bioassays in rats and mice, pyrazinamide was administered in the diet at concentrations of up to 10,000 ppm. This resulted in estimated daily doses for the mouse of 2 g/kg, or 40 times the maximum human dose, and for the rat of 0.5 g/kg, or 10 times the maximum human dose. Pyrazinamide was not carcinogenic in rats or male mice and no conclusion was possible for female mice due to insufficient numbers of surviving control mice.
Pyrazinamide was not mutagenic in the Ames bacterial test, but induced chromosomal aberrations in human lymphocyte cell cultures.
Pregnancy: Teratogenic Effects
Animal reproduction studies have not been conducted with pyrazinamide. It is also not known whether pyrazinamide can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Pyrazinamide should be given to a pregnant woman only if clearly needed.
Nursing Mothers:
Pyrazinamide has been found in small amounts in breast milk. Therefore, it is advised that pyrazinamide be used with caution in nursing mothers taking into account the risk-benefit of this therapy.9
Pediatric Use:
Pyrazinamide regimens employed in adults are probably equally effective in pediatric patients. 4,10,11
Pyrazinamide appears to be well tolerated in pediatric patients.
Geriatric Use: 12
Clinical studies of pyrazinamide did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger patients. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic or renal function, and of concomitant disease or other drug therapy.
It does not appear that patients with impaired renal function require a reduction in dose. It may be prudent to select doses at the low end of the dosing range, however.13
-
ADVERSE REACTIONS:
General:
Fever, porphyria and dysuria have rarely been reported. Gout (see PRECAUTIONS:).Gastrointestinal:
The principal adverse effect is a hepatic reaction (see WARNINGS:). Hepatotoxicity appears to be dose related, and may appear at any time during therapy. GI disturbances including nausea, vomiting and anorexia have also been reported.Hematologic and Lymphatic:
Thrombocytopenia and sideroblastic anemia with erythroid hyperplasia, vacuolation of erythrocytes and increased serum iron concentration have occurred rarely with this drug. Adverse effects on blood clotting mechanisms have also been rarely reported.Other:
Mild arthralgia and myalgia have been reported frequently. Hypersensitivity reactions including rashes, urticaria, and pruritis have been reported. Fever, acne, photosensitivity, porphyria, dysuria and interstitial nephritis have been reported rarely. - OVERDOSAGE:
-
DOSAGE & ADMINISTRATION:
Pyrazinamide should always be administered with other effective antituberculous drugs. It is administered for the initial 2 months of a 6-month or longer treatment regimen for drug-susceptible patients. Patients who are known or suspected to have drug-resistant disease should be treated with regimens individualized to their situation. Pyrazinamide frequently will be an important component of such therapy.
Patients with concomitant HIV infection may require longer courses of therapy. Physicians treating such patients should be alert to any revised recommendations from CDC for this group of patients.
Usual dose: Pyrazinamide is administered orally, 15 to 30 mg/kg once daily. Older regimens employed 3 or 4 divided doses daily, but most current recommendations are for once a day. Three grams per day should not be exceeded. The CDC recommendations do not exceed 2 g per day when given as a daily regimen (see table).
Alternatively, a twice weekly dosing regimen (50 to 75 mg/kg twice weekly based on lean body weight) has been developed to promote patient compliance with a regimen on an outpatient basis. In studies evaluating the twice weekly regimen, doses of pyrazinamide in excess of 3 g twice weekly have been administered. This exceeds the recommended maximum 3 g/daily dose. However, an increased incidence of adverse reactions has not been reported.
This table is taken from the CDC-American Thoracic Society joint recommendations.4
Recommended Drugs for the Initial Treatment of Tuberculosis in Children and Adults
Daily Dose*
Maximal Daily Dose in Children and Adults
Twice Weekly Dose
Drug
Children
Adults
Children
Adults
Isoniazid
10 to 20 mg/kg
PO or IM
5 mg/kg
PO or IM
300 mg
20 to 40 mg/kg
Max. 900 mg
15 mg/kg
Max. 900 mg
Rifampin
10 to 20 mg/kg
PO
10 mg/kg
PO
600 mg
10 to 20 mg/kg
Max. 600 mg
10 mg/kg
Max. 600 mg
Pyrazinamide
15 to 30 mg/kg
PO
15 to 30 mg/kg
PO
2 g
50 to 70 mg/kg
50 to 70 mg/kg
Streptomycin
20 to 40 mg/kg
IM
15 mg/kg**
IM
1 g**
25 to 30 mg/kg
IM
25 to 30 mg/kg
IM
Ethambutol
15 to 25 mg/kg
PO
15 to 25 mg/kg
PO
2.5 g
50 mg/kg
50 mg/kg
Definition of abbreviations: PO = perorally; IM = intramuscularly.
* Doses based on weight should be adjusted as weight changes.
**In persons older than 60 yrs of age the daily dose of streptomycin should be limited to 10 mg/kg with
a maximal dose of 750 mg. -
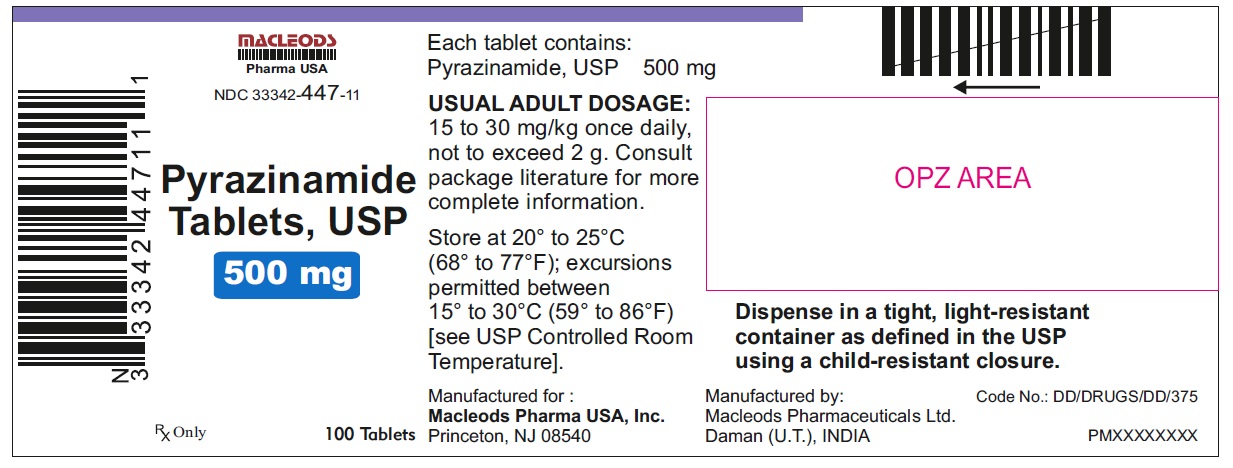
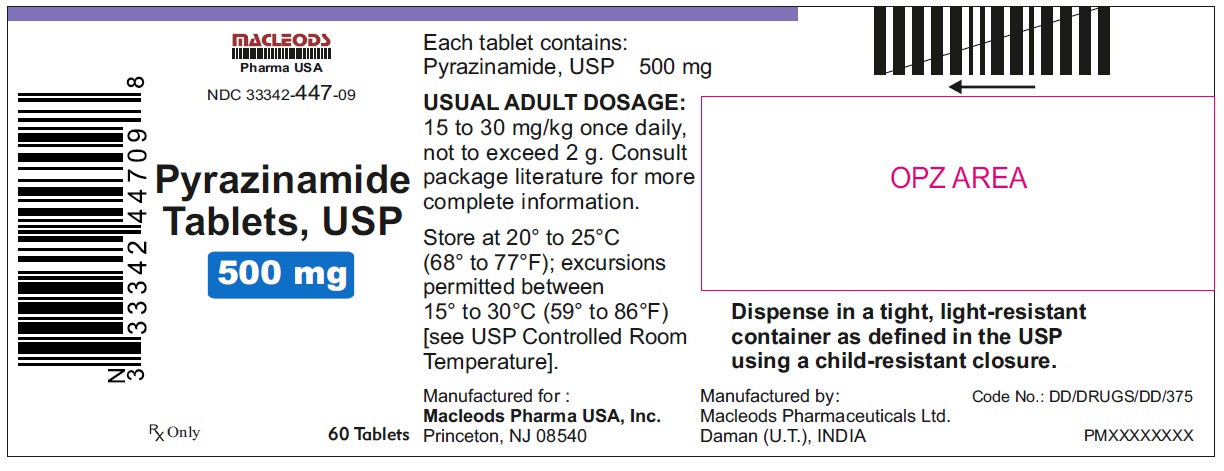
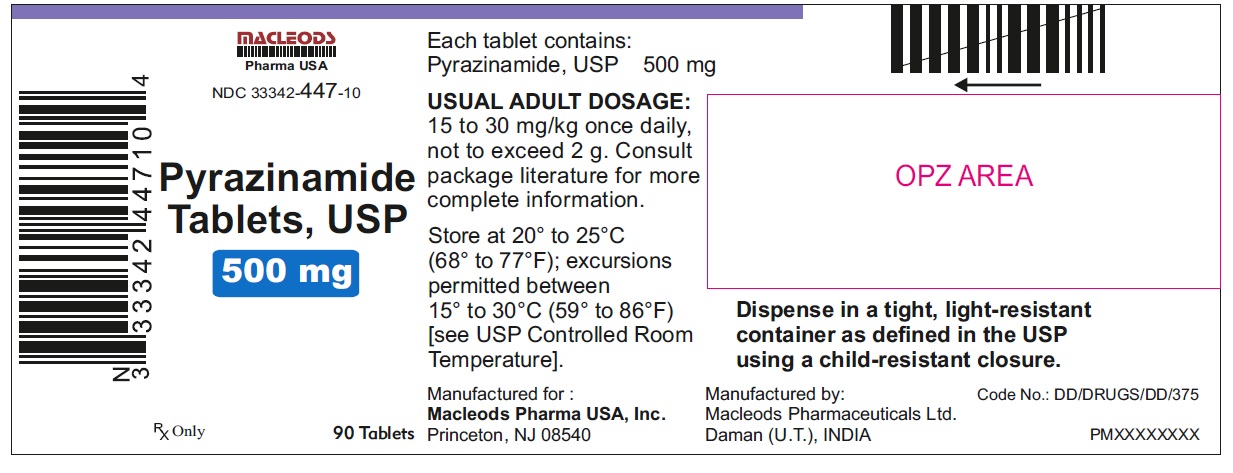
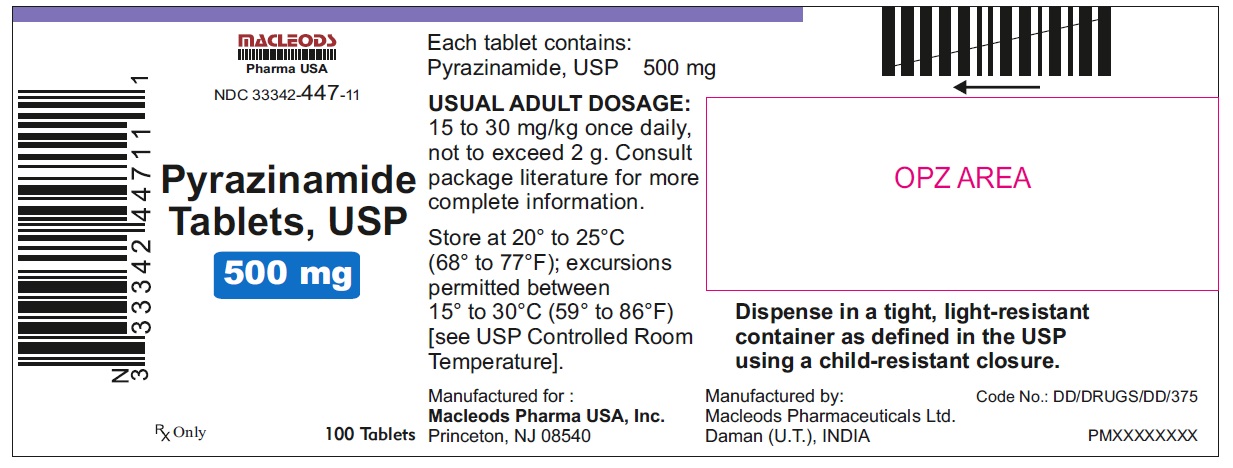
HOW SUPPLIED
Pyrazinamide Tablets, USP 500 mg are round, white to off white uncoated, scored tablets with notches, debossed "F" above the score and "43" below the score and plain on other side.
Tablets are supplied in the following strengths and package configurations:
Package configuration
NDC number
Bottles of 10
33342-447-03
Bottles of 30
33342-447-07
Bottles of 60
33342-447-09
Bottles of 90
33342-447-10
Bottles of 100
33342-447-11
Bottles of 500
33342-447-15
Carton of 100 Tablets
(10 x 10 unit dose)
33342-447-12
Store at 20° to 25° C (68° F to 77° F); excursions permitted between 15° to 30° C (59° F to 86° F) [see USP Controlled Room Temperature].
Dispense in a tight, light-resistant container as defined in the USP using a child-resistant closure.
Manufactured for:
Macleods Pharma USA, Inc.
Princeton, NJ 08540
Manufactured by:
Macleods Pharmaceutical Ltd.
Daman, (U.T.), INDIA
Revised: December 2022
-
REFERENCES
1.Drug Information, American Hospital Formulary Service. American Society of Hospital Pharmacists. Bethesda, Md. 1991.
2.USPDI, Drug Information for the Health Care Professional. United States Pharmacopeial Convention, Inc. Rockville, Md. 1991:1B:2226-2227.
3.Goodman-Gilman A, Rall TW, Nies AS, Taylor P. The Pharmacological Basis of Therapeutics, ed 8. New York, Pergamon Press. 1990;1154.
4.Treatment of tuberculosis and tuberculosis infection in adults and children. Am Rev Respir Dis.1986;134:363-368.
5.Reynolds JEF, Parfitt K, Parsons AV, Sweetman SC. Martindale The Extra Pharmacopoeia, ed 29. London, The Pharmaceutical Press. 1989;569-570.
6.Bioassay of pyrazinamide for possible carcinogenicity. National Cancer Institute Carcinogenesis Technical Report Series No. 48, 1978.
7.Zerger E, Anderson B, Haworth S, Lawlor T, Mortelmans K, Speck W. Salmonella mutagenicity tests: III. Results from the testing of 255 chemicals. Environ Mutagen. 1987;9 (Suppl 9):1109.
8.Roman IC, Georgian L. Cytogenetic effects of some antituberculosis drugs in vitro. Mutation Research. 1977;48:215-224.
9.Holdiness M. Antituberculosis drugs and breast-feeding. Arch Intern Med. 1984;144:1888.
10.Turcios N, Evans H. Preventing and managing tuberculosis in children. J Resp Dis. 1989;10(6)(Jun):23.
11.Starke JR. Multidrug therapy for tuberculosis in children. Pediatr Infec Dis J. 1990;9:785793.
12.Specific requirements on content and format of labeling for human prescription drugs; proposed addition of "geriatric use" subsection in the labeling. Federal Register. 1990;55(212)(Nov1):46134-46137.
13.Stamathakis G, Montes C, Trouvin JH, et al. Pyrazinamide and pyrazinoic acid pharmacokinetics in
patients with chronic renal failure. Clinical Nephrology. 1988;30:230-234.
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PYRAZINAMIDE
pyrazinamide tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:33342-447 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PYRAZINAMIDE (UNII: 2KNI5N06TI) (PYRAZINAMIDE - UNII:2KNI5N06TI) PYRAZINAMIDE 500 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) POVIDONE (UNII: FZ989GH94E) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) TALC (UNII: 7SEV7J4R1U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color WHITE (White to off white) Score 2 pieces Shape ROUND (Round uncoated scored tablets with notches) Size 12mm Flavor Imprint Code F;43 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:33342-447-03 10 in 1 BOTTLE; Type 0: Not a Combination Product 07/27/2020 2 NDC:33342-447-07 30 in 1 BOTTLE; Type 0: Not a Combination Product 07/27/2020 3 NDC:33342-447-09 60 in 1 BOTTLE; Type 0: Not a Combination Product 07/27/2020 4 NDC:33342-447-10 90 in 1 BOTTLE; Type 0: Not a Combination Product 07/27/2020 5 NDC:33342-447-11 100 in 1 BOTTLE; Type 0: Not a Combination Product 07/27/2020 6 NDC:33342-447-15 500 in 1 BOTTLE; Type 0: Not a Combination Product 07/27/2020 7 NDC:33342-447-12 10 in 1 BLISTER PACK; Type 0: Not a Combination Product 07/27/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA212541 07/27/2020 Labeler - Macleods Pharmaceuticals Limited (862128535) Establishment Name Address ID/FEI Business Operations Macleods Pharmaceuticals Limited 918608365 ANALYSIS(33342-447) , LABEL(33342-447) , MANUFACTURE(33342-447) , PACK(33342-447)