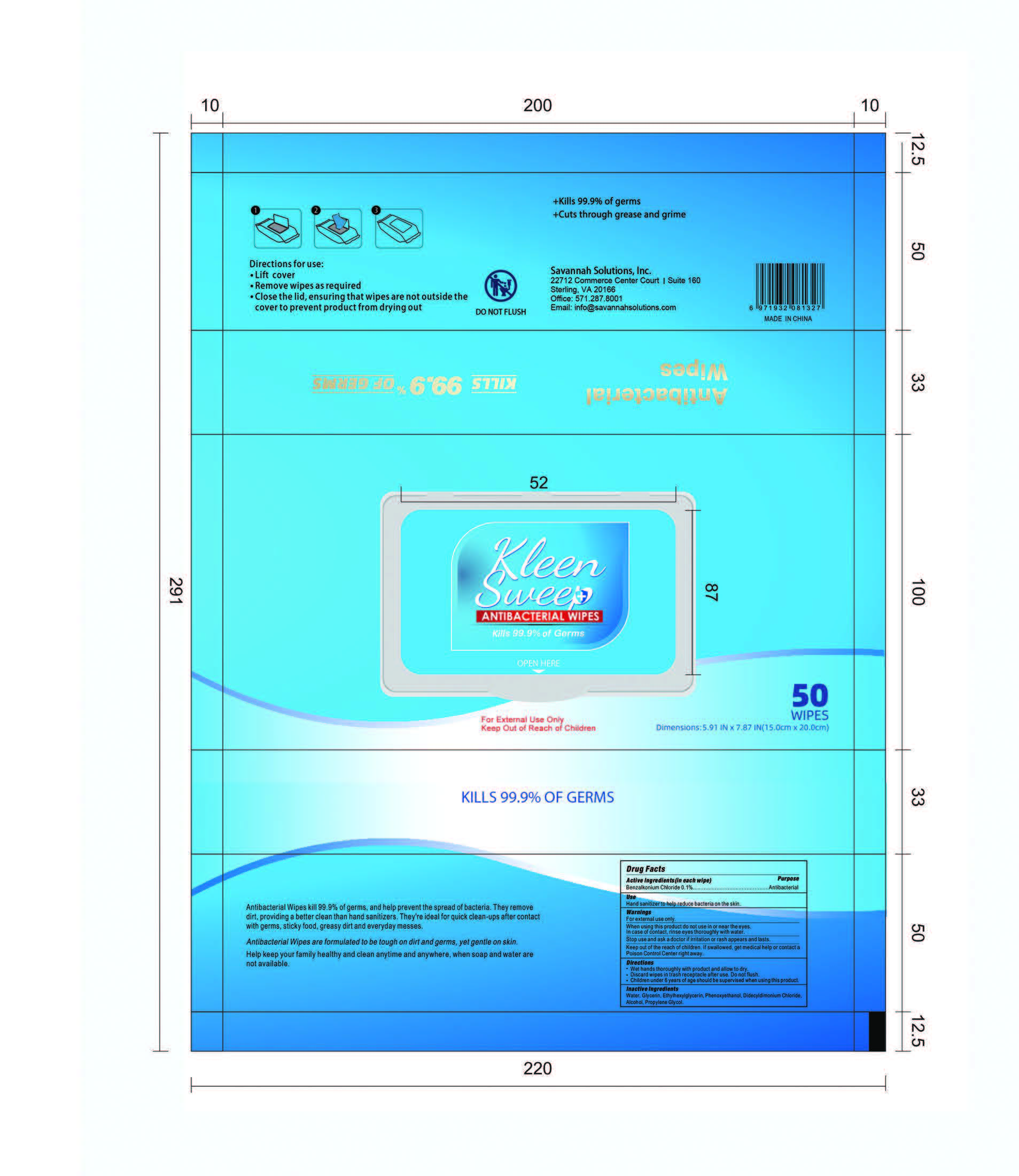
Label: KLEENSWEEP ANTIBACTERIAL WIPES- antibacterial wipes swab
-
Contains inactivated NDC Code(s)
NDC Code(s): 88888-001-50, 88888-001-70 - Packager: Savannah Solutions, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated June 20, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTION
Antibacterial wipes kill 99.9% of germs and help prevent the spread of bacterial. They remove dirt, providing a better clean than sanitizers. They are ideal for quick clean-ups after contact with germs, sticky food, greasy dirt, and everyday messes. Antibacterial wipes are formulated to be tough on dirt and germs, yet gentle on skin. Help keep your family healthy and clean anytime and anywhere when soap and water are not available.
The ingredients are as follows:
Benzalkonium Chloride :0.1%
Glycerin :0.1%
Ethylhexylglycerin :0.1%
Phenoxyethanol:0.5%
Didecyldimethylammonium Chloride :0.2%
Alcohol:10%
Propylene Glycol :0.1%
Water :88.9%.
- Active Ingredient(s)
- Purpose
- Use
- Warnings
-
Do not use
- Skin Contact: Skin irritation is not expected. Should irritation develop, discontinue use. Wash affected skin
thoroughly with soap and water. Get medical attention if irritation or other symptoms develop or persist.
- Eye Contact: Immediately flush eyes with plenty of water for at least 15 minutes, lifting lower and upper eyelids
occasionally. Get medical attention if irritation persists.
- Inhalation: Normal use of this product does not pose an inhalation hazard. Should respiratory tract irritation
develop, discontinue use and remove to fresh air. Get medical attention if irritation or other symptoms persist or
develop.- Ingestion: Seek medical attention immediately. Never give anything by mouth to an unconscious person. DO
NOT induce vomiting. If vomiting occurs, keep head below hips to prevent aspiration of liquid into lungs.
-
WHEN USING
- Skin Contact: Skin irritation is not expected. Should irritation develop, discontinue use. Wash affected skin
thoroughly with soap and water. Get medical attention if irritation or other symptoms develop or persist.
- Eye Contact: Immediately flush eyes with plenty of water for at least 15 minutes, lifting lower and upper eyelids
occasionally. Get medical attention if irritation persists.
Inhalation: Normal use of this product does not pose an inhalation hazard. Should respiratory tract irritation
develop, discontinue use and remove to fresh air. Get medical attention if irritation or other symptoms persist or
develop.- Ingestion: Seek medical attention immediately. Never give anything by mouth to an unconscious person. DO
NOT induce vomiting. If vomiting occurs, keep head below hips to prevent aspiration of liquid into lungs.
-
STOP USE
Emergency Overview:
WARNING! Liquid may cause irritation to the eyes. Prolonged or repeated contact may dry skin and cause
irritation and sensitization. Harmful if swallowed.Potential Health Effects
Route of entry: Inhalation, skin contact, eye contact, ingestion.
Skin Contact: Frequent or prolonged contact with skin may cause irritation.
Eye Contact: May cause eye irritation. Symptoms may include stinging, tearing and redness.
Inhalation: Not expected to be a significant exposure route. Vapor or mist may be irritating to mucous
membranes and upper respiratory tract.
Ingestion: May cause gastrointestinal irritation with nausea, vomiting and diarrhea. - KEEP OUT OF REACH OF CHILDREN
- Directions
- Other information
- Inactive ingredients
- Package Label - Principal Display Panel
-
INGREDIENTS AND APPEARANCE
KLEENSWEEP ANTIBACTERIAL WIPES
antibacterial wipes swabProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:88888-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.1 g in 100 g Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) DIDECYLDIMONIUM CHLORIDE (UNII: JXN40O9Y9B) PHENOXYETHANOL (UNII: HIE492ZZ3T) PROPYLENE GLYCOL 2-BEHENATE (UNII: 1L33JV6CWK) GLYCERIN (UNII: PDC6A3C0OX) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:88888-001-50 202.5 g in 1 BAG; Type 0: Not a Combination Product 07/01/2020 2 NDC:88888-001-70 264.6 g in 1 TUBE; Type 0: Not a Combination Product 07/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 07/01/2020 Labeler - Savannah Solutions, Inc. (024263561) Establishment Name Address ID/FEI Business Operations Zhejiang Ruolin Hygienic Products Co., Ltd. 415426870 manufacture(88888-001)