Label: PHENDIMETRAZINE TARTRATE tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 50090-2592-0, 50090-2592-1, 50090-2592-2, 50090-2592-6, view more50090-2592-9 - Packager: A-S Medication Solutions
- This is a repackaged label.
- Source NDC Code(s): 0185-4057
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CIII
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 25, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
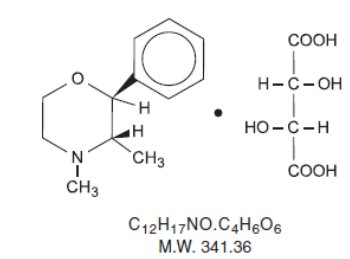
Phendimetrazine tartrate, as the dextro isomer, has the chemical name of (2s, 3s,)-3,4-dimethyl-2-phenylmorpholine L-(+)-tartrate (1:1).
The structural formula is as follows:
Phendimetrazine tartrate is a white, odorless crystalline powder. It is freely soluble in water; sparingly soluble in warm alcohol; insoluble in chloroform, acetone, ether and benzene.
Each tablet, for oral administration, contains 35 mg of phendimetrazine tartrate.
Inactive Ingredients: confectioner’s sugar (sucrose and corn starch), lactose monohydrate, povidone, pregelatinized starch, silicon dioxide and stearic acid.
The pink, white and blue tablets also contain: FD&C blue No. 1 and FD&C red No. 3.
The pink tablets also contain: FD&C red No. 3 and FD&C yellow No. 5 (see PRECAUTIONS).
The yellow tablets also contain: FD&C yellow No. 5 (see PRECAUTIONS).
-
CLINICAL PHARMACOLOGY
Phendimetrazine tartrate is a phenylalkylamine sympathomimetic amine with pharmacological activity similar to the prototype drugs of this class used in obesity, the amphetamines. Actions include central nervous system stimulation and elevation of blood pressure. Tachyphylaxis and tolerance have been demonstrated with all drugs of this class in which these phenomena have been looked for.
Drugs of this class used in obesity are commonly known as “anorectics” or “anorexigenics”. It has not been established, however, that the action of such drugs in treating obesity is primarily one of appetite suppression. Other central nervous system actions or metabolic effects, may be involved, for example.
Adult obese subjects instructed in dietary management and treated with “anorectic” drugs lose more weight on the average than those treated with placebo and diet, as determined in relatively short term clinical trials.
The magnitude of increased weight loss of drug-treated patients over placebo-treated patients is only a fraction of a pound a week. The rate of weight loss is greatest in the first weeks of therapy for both drug and placebo subjects and tends to decrease in succeeding weeks. The possible origins of the increased weight loss due to the various drug effects are not established. The amount of weight loss associated with the use of an anorectic drug varies from trial to trial and the increased weight loss appears to be related in part to variables other than the drug prescribed, such as the physician investigator, the population treated and the diet prescribed. Studies do not permit conclusions as to the relative importance of the drug and non-drug factors on weight loss.
The natural history of obesity is measured in years, whereas the studies cited are restricted to a few weeks duration, thus, the total impact of drug-induced weight loss over that of diet alone must be considered clinically limited.
-
INDICATIONS AND USAGE
Phendimetrazine tartrate tablets are indicated in the management of exogenous obesity as a short term adjunct (a few weeks) in a regimen of weight reduction based on caloric restriction. The limited usefulness of agents of this class (see CLINICAL PHARMACOLOGY) should be measured against possible risk factors inherent in their use such as those described below.
-
CONTRAINDICATIONS
Known hypersensitivity or idiosyncratic reactions to sympathomimetics.
Advanced arteriosclerosis, symptomatic cardiovascular disease, moderate and severe hypertension, hyperthyroidism and glaucoma.
Highly nervous or agitated patients.
Patients with a history of drug abuse.
Patients taking other CNS stimulants, including monoamine oxidase inhibitors.
-
WARNINGS
Tolerance to the anorectic effect of phendimetrazine develops within a few weeks. When this occurs, its use should be discontinued; the maximum recommended dose should not be exceeded.
Use of phendimetrazine tartrate within 14 days following the administration of monoamine oxidase inhibitors may result in a hypertensive crisis.
Abrupt cessation of administration following prolonged high dosage results in extreme fatigue and depression. Because of the effect on the central nervous system, phendimetrazine may impair the ability of the patient to engage in potentially hazardous activities such as operating machinery or driving a motor vehicle; the patient should therefore be cautioned accordingly.
-
PRECAUTIONS
Caution is to be exercised in prescribing phendimetrazine tartrate for patients with even mild hypertension.
Insulin requirements in diabetes mellitus may be altered in association with the use of phendimetrazine and the concomitant dietary regimen.
Phendimetrazine may decrease the hypotensive effect of guanethidine.
The least amount feasible should be prescribed or dispensed at one time in order to minimize the possibility of overdosage.
The phendimetrazine tartrate pink and yellow tablets contain FD&C yellow No. 5 (tartrazine) which may cause allergic type reactions (including bronchial asthma) in certain susceptible individuals. Although the overall incidence of FD&C yellow No. 5 (tartrazine) sensitivity in the general population is low, it is frequently seen in patients who also have aspirin hypersensitivity.
-
ADVERSE REACTIONS
Cardiovascular: Palpitation, tachycardia, elevated blood pressure.
Central Nervous System: Overstimulation, restlessness, insomnia, agitation, flushing, tremor, sweating, dizziness, headache, psychotic state, blurring of vision.
Gastrointestinal: Dryness of the mouth, nausea, diarrhea, constipation, stomach pain.
Genitourinary: Urinary frequency, dysuria, changes in libido.
-
DRUG ABUSE AND DEPENDENCE
Controlled Substance
Phendimetrazine tartrate tablets are defined by the Drug Enforcement Administration as a Schedule III controlled substance.
Dependence
Phendimetrazine tartrate is related chemically and pharmacologically to the amphetamines. Amphetamines and related stimulant drugs have been extensively abused and the possibility of abuse of phendimetrazine should be kept in mind when evaluating the desirability of including a drug as part of a weight reduction program. Abuse of amphetamines and related drugs may be associated with intense psychological dependence and severe social dysfunction. There are reports of patients who have increased the dosage to many times that recommended. Abrupt cessation following prolonged high dosage administration results in extreme fatigue and mental depression; changes are also noted on the sleep EEG. Manifestations of chronic intoxication with anorectic drugs include severe dermatoses, marked insomnia, irritability, hyperactivity and personality changes. The most severe manifestation of chronic intoxications is psychosis, often clinically indistinguishable from schizophrenia.
-
OVERDOSAGE
Acute overdosage with phendimetrazine tartrate may manifest itself by the following signs and symptoms: unusual restlessness, confusion, belligerence, hallucinations and panic states. Fatigue and depression usually follow the central stimulation. Cardiovascular effects include arrhythmias, hypertension or hypotension and circulatory collapse. Gastrointestinal symptoms include nausea, vomiting, diarrhea and abdominal cramps. Poisoning may result in convulsions, coma and death.
The management of overdosage is largely symptomatic. It includes sedation with a barbiturate. If hypertension is marked, the use of a nitrate or rapid-acting alpha receptor-blocking agent should be considered. Experience with hemodialysis or peritoneal dialysis is inadequate to permit recommendations for its use.
- DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- Storage
- Phendimetrazine Tartrate
-
INGREDIENTS AND APPEARANCE
PHENDIMETRAZINE TARTRATE
phendimetrazine tartrate tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:50090-2592(NDC:0185-4057) Route of Administration ORAL DEA Schedule CIII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHENDIMETRAZINE TARTRATE (UNII: 6985IP0T80) (PHENDIMETRAZINE - UNII:AB2794W8KV) PHENDIMETRAZINE TARTRATE 35 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STEARIC ACID (UNII: 4ELV7Z65AP) SUCROSE (UNII: C151H8M554) Product Characteristics Color YELLOW Score 2 pieces Shape ROUND Size 7mm Flavor Imprint Code E;76 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50090-2592-0 30 in 1 BOTTLE; Type 0: Not a Combination Product 12/07/2016 2 NDC:50090-2592-2 90 in 1 BOTTLE; Type 0: Not a Combination Product 09/08/2017 3 NDC:50090-2592-6 28 in 1 BOTTLE; Type 0: Not a Combination Product 08/10/2017 4 NDC:50090-2592-9 112 in 1 BOTTLE; Type 0: Not a Combination Product 02/03/2017 5 NDC:50090-2592-1 60 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/28/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA085588 08/19/1977 Labeler - A-S Medication Solutions (830016429) Establishment Name Address ID/FEI Business Operations A-S Medication Solutions 830016429 RELABEL(50090-2592) , REPACK(50090-2592)