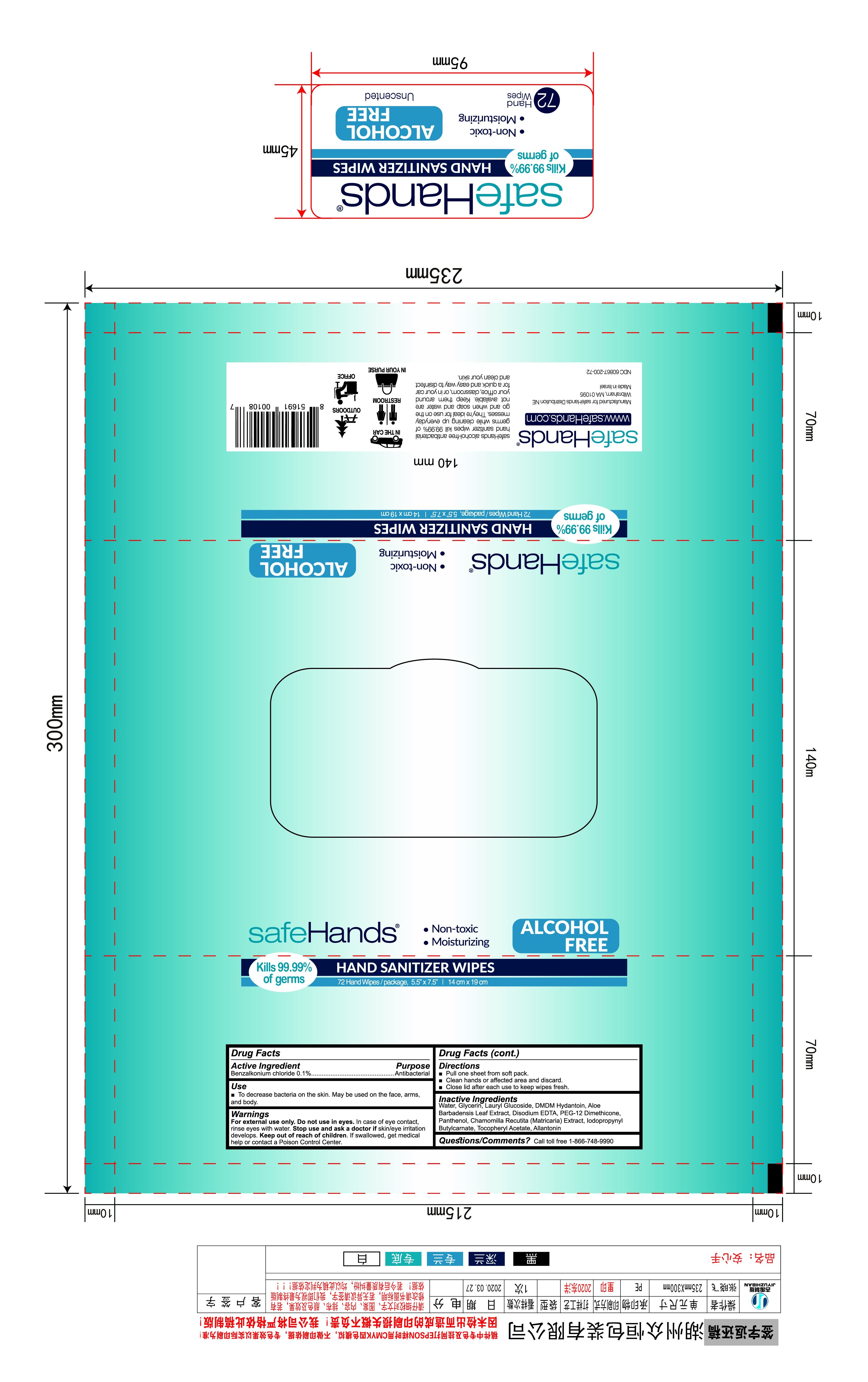
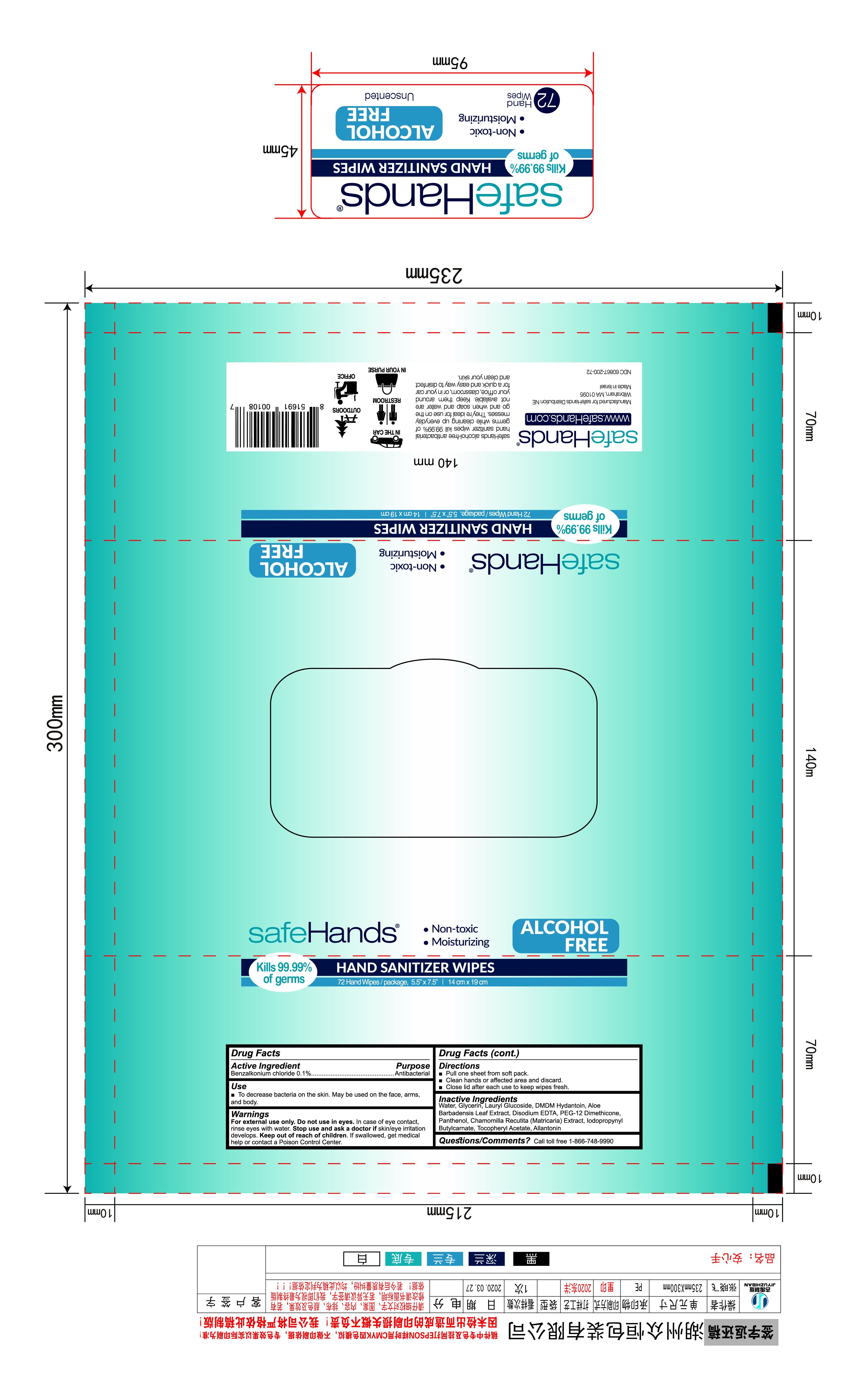
Label: HAND SANITIZER WIPES- wet wipe swab
- NDC Code(s): 77046-003-01
- Packager: ZHEJIANG HUASHUN TECHNOLOGY CO.,LTD
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Use
- Warnings
- Active Ingredient
- Inactive Ingredient
- Questions/Comments?
- PURPOSE
- Directions
- KEEP OUT OF REACH OF CHILDREN
- package label
-
INGREDIENTS AND APPEARANCE
HAND SANITIZER WIPES
wet wipe swabProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:77046-003 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.1 g in 100 g Inactive Ingredients Ingredient Name Strength ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) 0.05 g in 100 g DECYL GLUCOSIDE (UNII: Z17H97EA6Y) 0.05 g in 100 g ALOE (UNII: V5VD430YW9) 0.05 g in 100 g WATER (UNII: 059QF0KO0R) PHENOXYETHANOL (UNII: HIE492ZZ3T) 0.5 g in 100 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:77046-003-01 72 in 1 BAG 04/30/2020 1 4.15 g in 1 PATCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 04/30/2020 Labeler - ZHEJIANG HUASHUN TECHNOLOGY CO.,LTD (527157837) Establishment Name Address ID/FEI Business Operations ZHEJIANG HUASHUN TECHNOLOGY CO., LTD 527157837 manufacture(77046-003)