Label: WONVIA ANTIBACTERIAL WIPES- benzalkonium chloride cloth
-
Contains inactivated NDC Code(s)
NDC Code(s): 78876-707-12, 78876-707-72 - Packager: Kaplan Distribution LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated August 12, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DESCRIPTION
- Active Ingredient(s)
-
Inactive ingredients
Inactive material name: Aqua, Olus Oil, Lauryl Glucoside, Glycerin, Propylene Glycol, Polyglyceryl-2 Dipolyhydroxystearate, Phenoxyethanol, Parfum, Polysorbate 20, Glyceryl Oleate, Dicaprylyl Carbonate, Dehdroacetic Acid, Benzoic Acid, Tetrasodium EDTA, Benzyl Alcohol, Benzyl Salicylate, Citronellol, Coumarin, Linalool.
- Purpose
- DOSAGE & ADMINISTRATION
- Use
- Warnings
- Do not use
- STORAGE AND HANDLING
- KEEP OUT OF REACH OF CHILDREN
- DISPOSAL AND WASTE HANDLING
- QUESTIONS
-
Package Label - Principal Display Panel
WONVIA antibacterial
antibacterial wipes
kills 99,9% of bacteria
120 wipes
effective hygiene wipes
Use biocides safely and sustainably.
Production date, expiry date and batch number are on the pack.
Kaplan Distribution LLC
828 N. OLDEN AVE. TRENTON, NJ 08638 USA
Made in Turkey
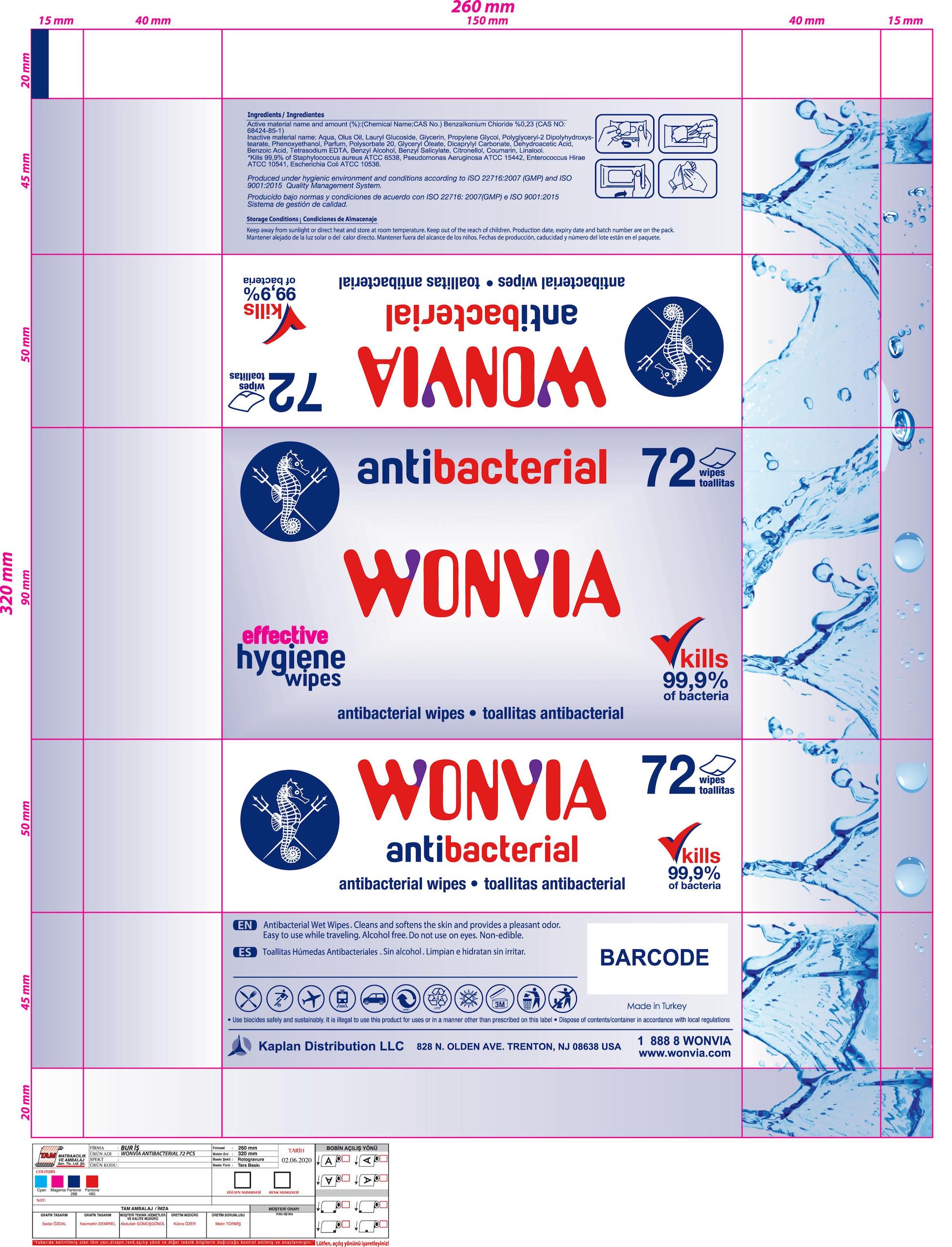

Produced under hygienic environment and conditions according to ISO 22716:2007 (GMP) and ISO 9001:2015 Quality Management System.
-
INGREDIENTS AND APPEARANCE
WONVIA ANTIBACTERIAL WIPES
benzalkonium chloride clothProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:78876-707 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.23 g in 100 g Inactive Ingredients Ingredient Name Strength POLYSORBATE 20 (UNII: 7T1F30V5YH) BENZOIC ACID (UNII: 8SKN0B0MIM) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) BENZYL ALCOHOL (UNII: LKG8494WBH) COUMARIN (UNII: A4VZ22K1WT) LINALOOL, (+)- (UNII: F4VNO44C09) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) EDETATE SODIUM (UNII: MP1J8420LU) BENZYL SALICYLATE (UNII: WAO5MNK9TU) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) LAURYL GLUCOSIDE (UNII: 76LN7P7UCU) POLYGLYCERYL-2 DIPOLYHYDROXYSTEARATE (UNII: 9229XJ4V12) PHENOXYETHANOL (UNII: HIE492ZZ3T) GLYCERYL OLEATE (UNII: 4PC054V79P) DEHYDROACETIC ACID (UNII: 2KAG279R6R) Product Characteristics Color Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:78876-707-72 335 g in 1 PACKAGE; Type 0: Not a Combination Product 06/16/2020 2 NDC:78876-707-12 460 g in 1 PACKAGE; Type 0: Not a Combination Product 06/16/2020 
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 06/16/2020 Labeler - Kaplan Distribution LLC (117548994) Registrant - HARDAL GLOBAL CONSULTING LLC (123584960) Establishment Name Address ID/FEI Business Operations KAPLAN DISTRIBUTION LLC 117548994 label(78876-707) Establishment Name Address ID/FEI Business Operations RKS GROUP YAPI KIMYASALLARI SANAYI VE TICARET LIMITED SIRKETI 520136931 manufacture(78876-707)


