Label: MECLIZINE HCL- meclizine hydrochloride chewable tablet, chewable
- NDC Code(s): 68788-8529-1, 68788-8529-3
- Packager: Preferred Pharmaceuticals Inc.
- This is a repackaged label.
- Source NDC Code(s): 16571-824
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated July 2, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts Active ingredient (in each chewable tablet)
- Purpose
- Uses
- Warnings
- ASK DOCTOR/PHARMACIST
- When using this product
- PREGNANCY OR BREAST FEEDING
- Keep out of reach of children.
- Directions
- Other Information
- Inactive ingredients
- Questions or comments?
- TAMPER EVIDENT: DO NOT USE IF FOIL SEAL UNDER CAP, PRINTED WITH"SEALED for YOUR PROTECTION" IS BROKEN OR MISSING.Rising Pharma Holdings, Inc. is not affiliated with the owner of the registered trademark Bonine® Manufactured by: Unique Pharmaceutical Laboratories(A Div. of J.B. Chemicals & Pharmaceuticals Ltd.),Mumbai 400 030, India Distributed by:Rising Pharma Holdings, Inc.East Brunswick, NJ 08816 Mfg. Lic. No.: G/1430Feb 2022
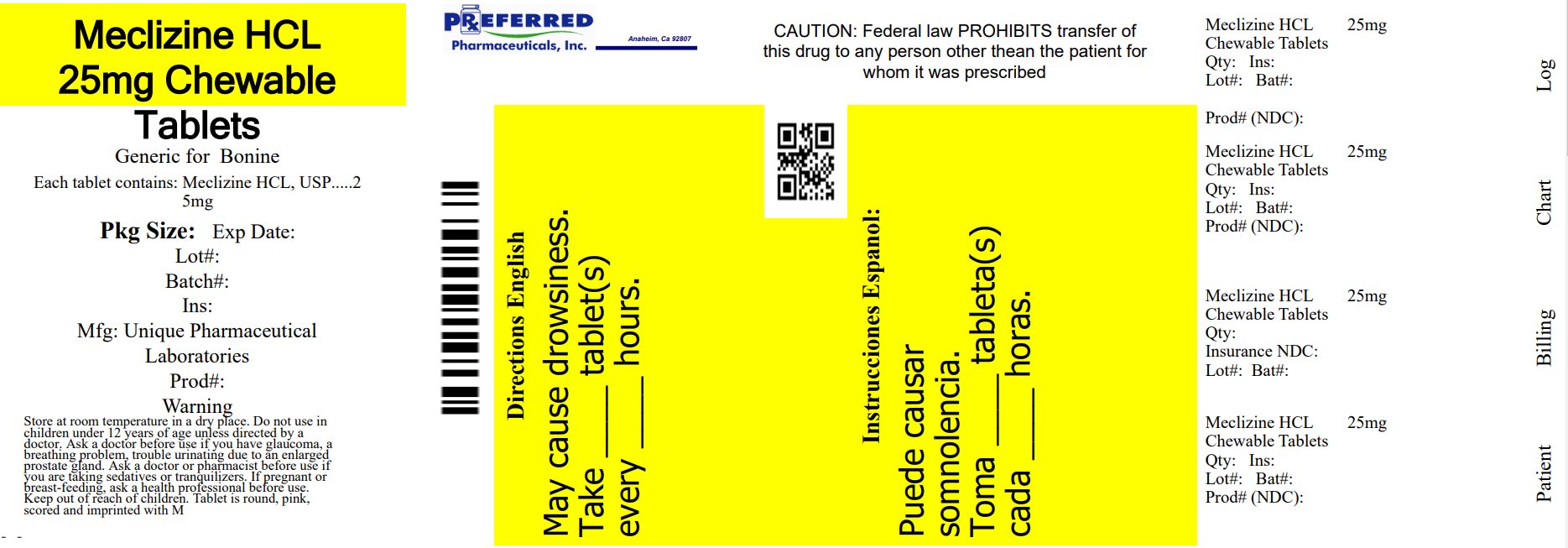
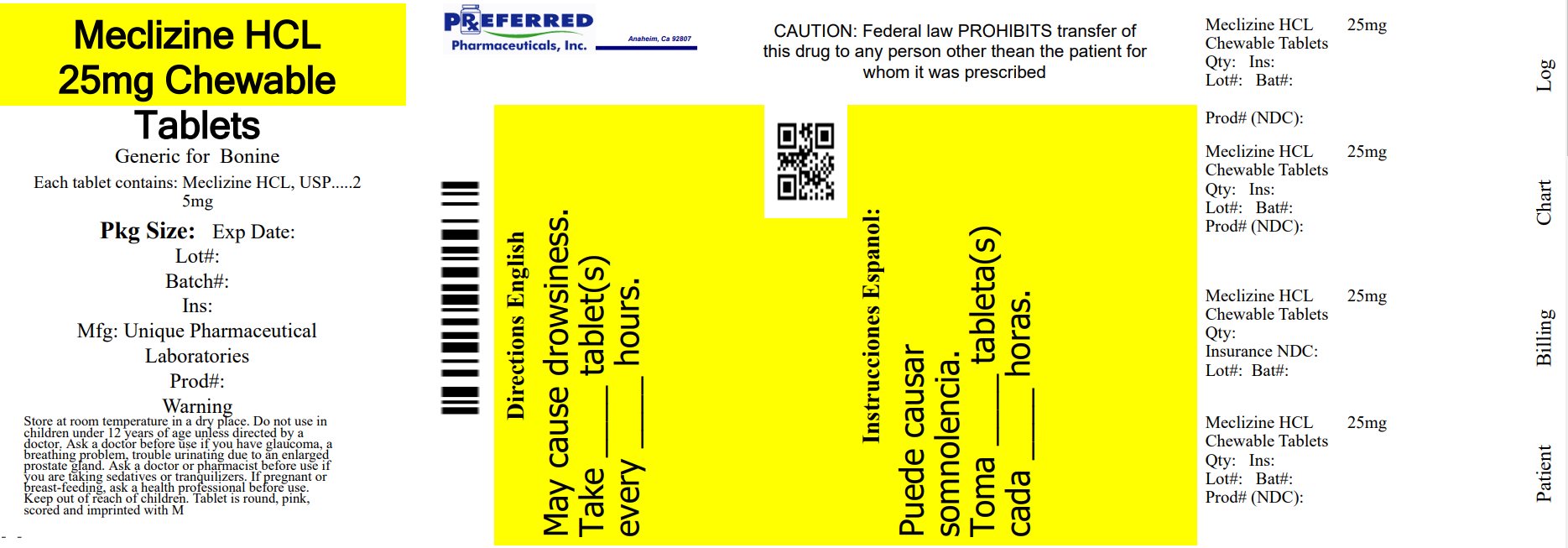
- PRINCIPAL DISPLAY PANEL - 25 mg Chewable Tablet Label
-
INGREDIENTS AND APPEARANCE
MECLIZINE HCL
meclizine hydrochloride chewable tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68788-8529(NDC:16571-824) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MECLIZINE HYDROCHLORIDE (UNII: HDP7W44CIO) (MECLIZINE - UNII:3L5TQ84570) MECLIZINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength ASPARTAME (UNII: Z0H242BBR1) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) DEXTROSE, UNSPECIFIED FORM (UNII: IY9XDZ35W2) FD&C RED NO. 40 (UNII: WZB9127XOA) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) RASPBERRY (UNII: 4N14V5R27W) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM SULFATE ANHYDROUS (UNII: 36KCS0R750) SUCROSE (UNII: C151H8M554) TRIBASIC CALCIUM PHOSPHATE (UNII: 91D9GV0Z28) Product Characteristics Color pink (Pink to light pink) Score 2 pieces Shape ROUND Size 8mm Flavor RASPBERRY Imprint Code M Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68788-8529-1 10 in 1 BOTTLE; Type 0: Not a Combination Product 10/06/2023 2 NDC:68788-8529-3 30 in 1 BOTTLE; Type 0: Not a Combination Product 10/06/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 336 10/06/2023 Labeler - Preferred Pharmaceuticals Inc. (791119022) Registrant - Preferred Pharmaceuticals Inc. (791119022) Establishment Name Address ID/FEI Business Operations Preferred Pharmaceuticals Inc. 791119022 REPACK(68788-8529)