Label: OSCEOLA SUPPLY INC 6176- chloroxylenol soap
-
NDC Code(s):
62672-300-01,
62672-300-03,
62672-300-05,
62672-300-07, view more62672-300-09, 62672-300-10, 62672-300-11, 62672-300-12, 62672-300-13, 62672-300-14, 62672-300-15, 62672-300-16, 62672-300-17, 62672-300-18, 62672-300-19, 62672-300-20, 62672-300-24, 62672-300-27, 62672-300-28, 62672-300-35, 62672-300-55
- Packager: OSCEOLA SUPPLY, INC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated July 22, 2016
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts Box OTC-Active Ingredient Section
- Drug Facts Box OTC-Purpose Section
- Drug Facts Box OTC-Indications & Usage Section
- Drug Facts Box OTC-Warnings Section
- Drug Facts Box OTC-When Using Section
- Drug Facts Box OTC-Stop Use Section
- Drug Facts Box OTC-Keep Out of Reach of Children Section
- Drug Facts Box OTC-Dosage & Administration Section
- Drug Facts Box OTC-Inactive Ingredient Section
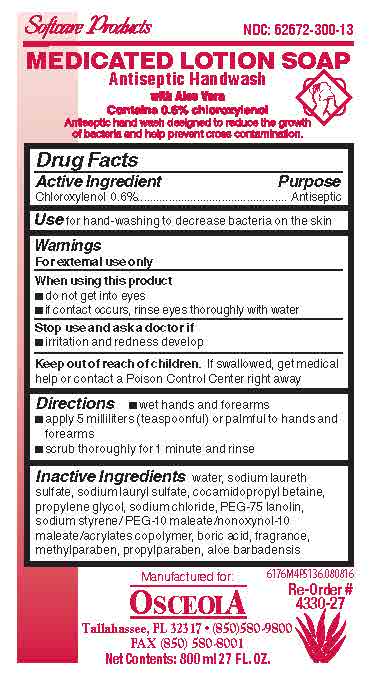
- Medicated Lotion Soap 6176 Label
-
INGREDIENTS AND APPEARANCE
OSCEOLA SUPPLY INC 6176
chloroxylenol soapProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62672-300 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLOROXYLENOL (UNII: 0F32U78V2Q) (CHLOROXYLENOL - UNII:0F32U78V2Q) CHLOROXYLENOL 6 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SODIUM LAURETH SULFATE (UNII: BPV390UAP0) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SODIUM CHLORIDE (UNII: 451W47IQ8X) ALOE VERA LEAF (UNII: ZY81Z83H0X) PROPYLPARABEN (UNII: Z8IX2SC1OH) METHYLPARABEN (UNII: A2I8C7HI9T) BORIC ACID (UNII: R57ZHV85D4) SODIUM LAURYL SULFATE (UNII: 368GB5141J) PEG-75 LANOLIN (UNII: 09179OX7TB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62672-300-17 532 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/22/2016 2 NDC:62672-300-24 118 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/22/2016 3 NDC:62672-300-01 1200 mL in 1 CARTRIDGE; Type 0: Not a Combination Product 07/22/2016 4 NDC:62672-300-03 350 mL in 1 CARTRIDGE; Type 0: Not a Combination Product 07/22/2016 5 NDC:62672-300-05 540 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/22/2016 6 NDC:62672-300-07 700 mL in 1 BAG; Type 0: Not a Combination Product 07/22/2016 7 NDC:62672-300-09 2000 mL in 1 CARTRIDGE; Type 0: Not a Combination Product 07/22/2016 8 NDC:62672-300-10 1000 mL in 1 CARTRIDGE; Type 0: Not a Combination Product 07/22/2016 9 NDC:62672-300-11 1000 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/22/2016 10 NDC:62672-300-12 1000 mL in 1 BAG; Type 0: Not a Combination Product 07/22/2016 11 NDC:62672-300-13 800 mL in 1 BAG; Type 0: Not a Combination Product 07/22/2016 12 NDC:62672-300-14 3785 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/22/2016 13 NDC:62672-300-15 946 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/22/2016 14 NDC:62672-300-28 149 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/22/2016 15 NDC:62672-300-27 800 mL in 1 CARTRIDGE; Type 0: Not a Combination Product 07/22/2016 16 NDC:62672-300-55 2082 mL in 1 DRUM; Type 0: Not a Combination Product 07/22/2016 17 NDC:62672-300-16 236 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/22/2016 18 NDC:62672-300-18 50 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/22/2016 19 NDC:62672-300-19 1890 mL in 1 CONTAINER; Type 0: Not a Combination Product 07/22/2016 20 NDC:62672-300-20 7560 mL in 1 DRUM; Type 0: Not a Combination Product 07/22/2016 21 NDC:62672-300-35 1325 mL in 1 DRUM; Type 0: Not a Combination Product 07/22/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 07/22/2016 Labeler - OSCEOLA SUPPLY, INC. (809050479) Registrant - ABC Compounding Co., Inc. (003284353) Establishment Name Address ID/FEI Business Operations ABC Compounding Co., Inc. 003284353 manufacture(62672-300)

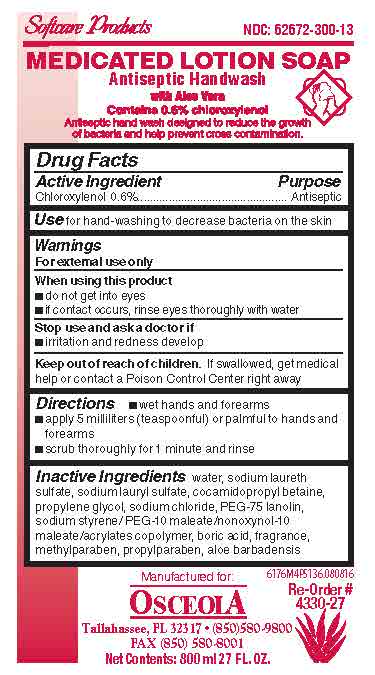
 6176M4P5136.jpg
6176M4P5136.jpg