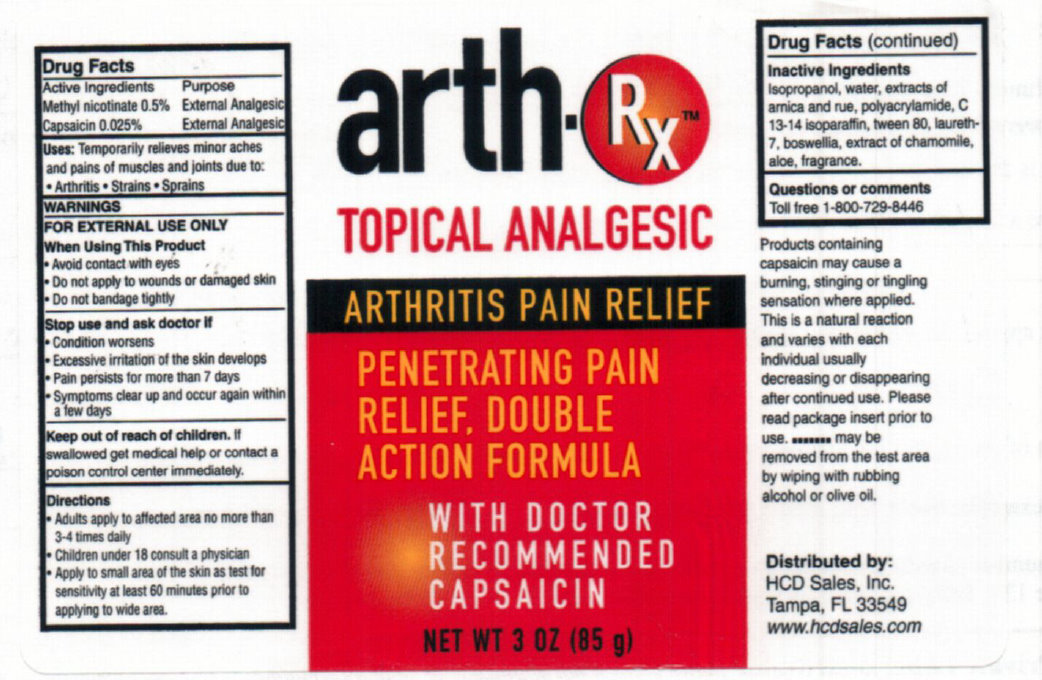
Label: ARTH RX TOPICAL ANALGESIC- methyl nicotinate, capsaicin lotion
- NDC Code(s): 76069-100-03
- Packager: HCD Sales
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 6, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- WARNINGS
- INDICATIONS & USAGE
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- INACTIVE INGREDIENT
- QUESTIONS
-
DESCRIPTION
Products containing capsaicin may cause a burning, stinging or tingling sensation where applied. This is a natural reaction and varies with each individual usually decreasing or disappearing after continued use. Please read package insert prior to use. .......... may be removed from the test area by wiping or olive oil.
Distributed by: HCD sales, Inc, Tampa, FL 33549 www.hcdsales.com
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ARTH RX TOPICAL ANALGESIC
methyl nicotinate, capsaicin lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76069-100 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METHYL NICOTINATE (UNII: 7B1AVU9DJN) (NIACIN - UNII:2679MF687A) METHYL NICOTINATE 0.5 g in 100 g CAPSAICIN (UNII: S07O44R1ZM) (CAPSAICIN - UNII:S07O44R1ZM) CAPSAICIN 0.025 g in 100 g Inactive Ingredients Ingredient Name Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) WATER (UNII: 059QF0KO0R) POLYQUATERNIUM-7 (70/30 ACRYLAMIDE/DADMAC; 1600 KD) (UNII: 0L414VCS5Y) C13-14 ISOPARAFFIN (UNII: E4F12ROE70) POLYSORBATE 80 (UNII: 6OZP39ZG8H) LAURETH-7 (UNII: Z95S6G8201) ALOE (UNII: V5VD430YW9) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76069-100-03 85 g in 1 BOTTLE; Type 0: Not a Combination Product 02/05/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 02/12/2011 Labeler - HCD Sales (137317314)