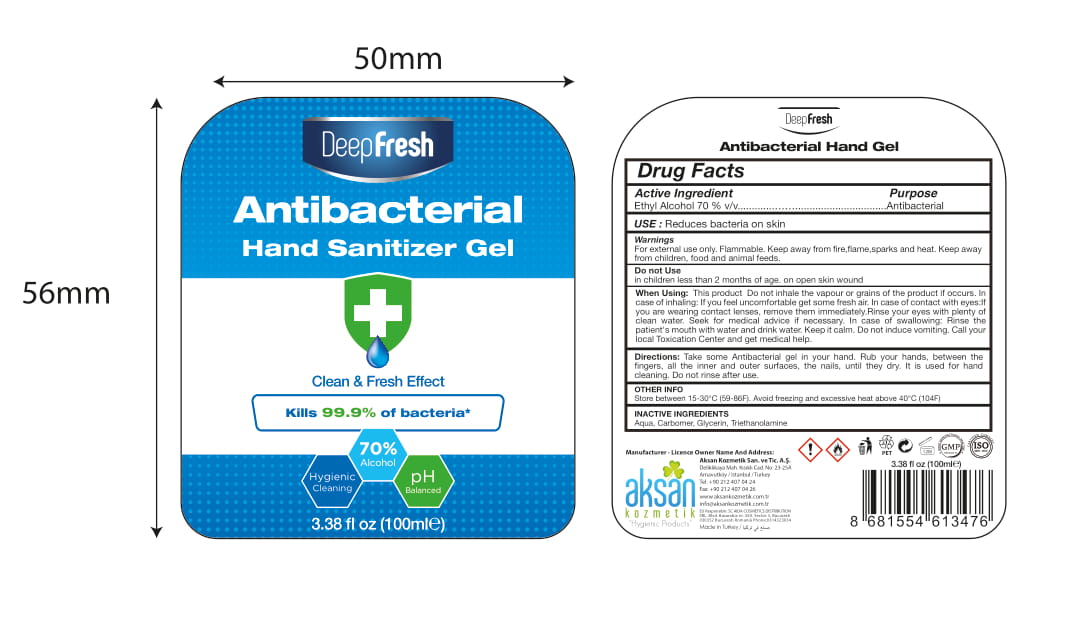
Label: DEEP FRESH ANTIBACTERIAL HAND SANITIZER GEL- alcohol gel
-
NDC Code(s):
86815-002-01,
86815-002-02,
86815-002-03,
86815-002-04, view more86815-002-05
- Packager: AKSAN KOZMETIK SANAYI VE TICARET ANONIM SIRKETI
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 23, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTION
This is a hand sanitizer manufactured according to the Temporary Policy for Preparation of Certain Alcohol-Based Hand Sanitizer Products During the Public Health Emergency (CoViD-19); Guidance for Industry.
The hand sanitizer is manufactured using only the following United States Pharmacopoeia (USP) grade ingredients in the preparation of the product (percentage in final product formulation) consistent with World Health Organization (WHO) recommendations:
- Alcohol (ethanol) (USP or Food Chemical Codex (FCC) grade) 70%, volume/volume (v/v)) in an aqueous solution denatured according to Alcohol and Tobacco Tax and Trade Bureau regulations in 27 CFR part 20.
- Glycerol (0.5% v/v).
- Carbomer (0.35% v/v).
- Triethanolamine (0.1% v/v).
- Sterile distilled water or boiled cold water.
The firm does not add other active or inactive ingredients. Different or additional ingredients may impact the quality and potency of the product.
- Active Ingredient(s)
- Purpose
- Use
- Warnings
- Do not use
-
WHEN USING
Do not inhale the vapour or grains of the product if occurs. In case of inhaling: If you feel uncomfortable get some fresh air.In case of contact with eyes:If you are wearing contact lenses, remove them immediately.Rinse your eyes with plenty of clean water. Seek for medical advice if necessary. In case of swallowing: Rinse the patient's mouth with water and drink water. Keep it calm. Do not induce vomiting. Call your local Toxication Center and get medical help.
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other information
- Inactive ingredients
- Package Label - Principal Display Panel
-
INGREDIENTS AND APPEARANCE
DEEP FRESH ANTIBACTERIAL HAND SANITIZER GEL
alcohol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:86815-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 70 mL in 100 mL Inactive Ingredients Ingredient Name Strength CARBOMER 1342 (UNII: 809Y72KV36) 0.35 mL in 100 mL GLYCERIN (UNII: PDC6A3C0OX) 0.5 mL in 100 mL TROLAMINE (UNII: 9O3K93S3TK) 0.1 mL in 100 mL WATER (UNII: 059QF0KO0R) 29.05 mL in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:86815-002-04 1000 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 03/30/2020 2 NDC:86815-002-05 2500 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 03/30/2020 3 NDC:86815-002-01 500 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 03/30/2020 4 NDC:86815-002-02 100 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 03/30/2020 5 NDC:86815-002-03 200 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 03/30/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 03/30/2020 Labeler - AKSAN KOZMETIK SANAYI VE TICARET ANONIM SIRKETI (365577706) Registrant - AKSAN KOZMETIK SANAYI VE TICARET ANONIM SIRKETI (365577706) Establishment Name Address ID/FEI Business Operations AKSAN KOZMETIK SANAYI VE TICARET ANONIM SIRKETI 365577706 manufacture(86815-002)