Label: BACITRACIN ZINC AND POLYMYXIN B SULFATE ointment
- NDC Code(s): 68071-5267-3
- Packager: NuCare Pharmaceuticals,Inc.
- This is a repackaged label.
- Source NDC Code(s): 24208-555
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 6, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Bacitracin Zinc and Polymyxin B Sulfate Ophthalmic Ointment, USP is a sterile antimicrobial ointment formulated for ophthalmic use.
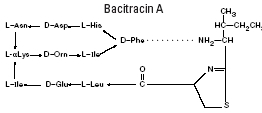
Bacitracin zinc is the zinc salt of bacitracin, a mixture of related cyclic polypeptides (mainly bacitracin A) produced by the growth of an organism of the licheniformis group of Bacillus subtilis var Tracy. It has a potency of not less than 40 bacitracin units/mg. The structural formula for bacitracin A is:
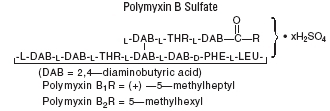
Polymyxin B sulfate is the sulfate salt of polymyxin B 1 and B 2, which are produced by the growth of Bacillus polymyxa (Prazmowski) Migula (Fam. Bacillaceae). It has a potency of not less than 6,000 polymyxin B units/mg, calculated on an anhydrous basis. The structural formulae are:
Each gram contains: Actives: Bacitracin Zinc equal to 500 bacitracin units and Polymyxin B Sulfate equal to 10,000 polymyxin B units; Inactives: Mineral Oil and White Petrolatum.
- CLINICAL PHARMACOLOGY
- INDlCATlONS AND USAGE
- CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- ADVERSE REACTIONS
- DOSAGE AND ADMINISTRATION
-
HOW SUPPLIED
Bacitracin Zinc and Polymyxin B Sulfate Ophthalmic Ointment, USP is available in tubes with an ophthalmic tip applicator in the following size:
Box of 3.5g NDC 68071-5267-3
Storage
Store between 15° to 25°C (59° to 77°F). KEEP TIGHTLY CLOSED
Keep out of reach of children.
Distributed by:
Bausch + Lomb, a division of Bausch Health US, LLC
Bridgewater, NJ 08807 USA
Manufactured by:
Bausch & Lomb Incorporated
Tampa, FL 33637 USA
© 2020 Bausch & Lomb Incorporated or its affiliates
Revised: February 2020
9130703 (Folded)
9130603 (Flat)
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BACITRACIN ZINC AND POLYMYXIN B SULFATE
bacitracin zinc and polymyxin b sulfate ointmentProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:68071-5267(NDC:24208-555) Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BACITRACIN ZINC (UNII: 89Y4M234ES) (BACITRACIN - UNII:58H6RWO52I) BACITRACIN 500 [USP'U] in 1 g POLYMYXIN B SULFATE (UNII: 19371312D4) (POLYMYXIN B - UNII:J2VZ07J96K) POLYMYXIN B 10000 [USP'U] in 1 g Inactive Ingredients Ingredient Name Strength MINERAL OIL (UNII: T5L8T28FGP) PETROLATUM (UNII: 4T6H12BN9U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68071-5267-3 3.5 g in 1 BOX; Type 0: Not a Combination Product 06/03/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA064046 04/25/2008 Labeler - NuCare Pharmaceuticals,Inc. (010632300) Establishment Name Address ID/FEI Business Operations NuCare Pharmaceuticals,Inc. 010632300 relabel(68071-5267)