Label: VISBELLA HYPOCHLOROUS ACID DISINFECTANT liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 40760-013-01 - Packager: Huzhou Guoneng New Material Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated June 3, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DOSAGE & ADMINISTRATION
- INACTIVE INGREDIENT
-
INDICATIONS & USAGE
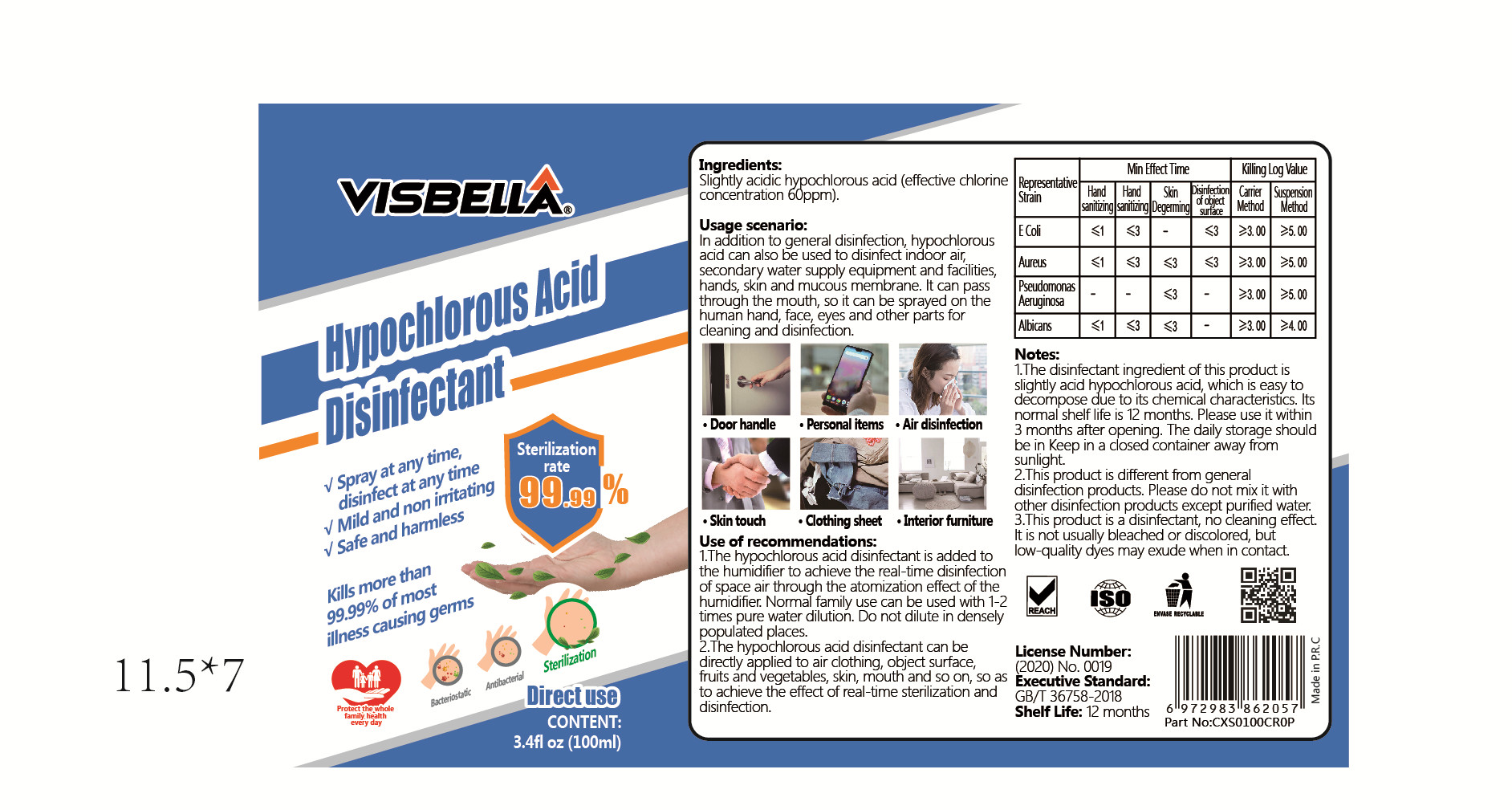
1.The hypochlorous acid disinfectant is added to the humidifer to achieve the real-time disinfection of space air through the atomization effect of the humidifier. Normal family use can be used with 1-2 times pure water dilution. Do not dilute in densely populated places.
2.The hypochlorous acid disinfectant can be directly applied to air clothing, object surface,fruits and vegetables, skin, mouthi and so on, so as to achieve the effect of real-time sterilization and disinfection. - ACTIVE INGREDIENT
- KEEP OUT OF REACH OF CHILDREN
- PURPOSE
-
WARNINGS
1.The disinfectant ingredient of this product is slightly acid hypochlorous acid, which is easy to decompose due to its chemical characteristics. lts normal shelf life is 12 months. Please use it within 3 months after opening. The daily storage should be in Keep in a closed container away from sunlight.
2.This product is different from general disinfection products. Please do not mix it with other disinfection products except purifed water.
3.This product is a disinfectant, no cleaning effect.It is not usually bleached or discolored, but low-quality dyes may exude when in contact. - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
VISBELLA HYPOCHLOROUS ACID DISINFECTANT
visbella hypochlorous acid disinfectant liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:40760-013 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYPOCHLOROUS ACID (UNII: 712K4CDC10) (HYPOCHLOROUS ACID - UNII:712K4CDC10) HYPOCHLOROUS ACID 6 mg in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Product Characteristics Color Score Shape Size Flavor Imprint Code VISBELLA Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:40760-013-01 100 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/03/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 06/03/2020 Labeler - Huzhou Guoneng New Material Co., Ltd. (407609050) Establishment Name Address ID/FEI Business Operations Huzhou Guoneng New Material Co., Ltd. 407609050 manufacture(40760-013)