Label: HUMATIN- paromomycin sulfate capsule
- NDC Code(s): 80725-250-01
- Packager: Waylis Therapeutics LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 10, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Paromomycin sulfate is a broad spectrum antibiotic produced by Streptomyces riomosus var. paromomycinus. It is a white, amorphous, stable, water-soluble product. Paromomycin sulfate is designated chemically as 0-2, 6-Diamino-2, 6-dideoxy-β -L-idopyranosyl-(1→3)-0-β -D-ribofuranosyl-(1→5)-0-[2-amino-2-deoxy-α -D-glucopyranosyl-(1→4)]-2-deoxystreptamine sulfate (salt). The molecular formula is C23H45N5O14•xH2SO4, with a molecular weight of 615.64 (base).
Its structural formula is:
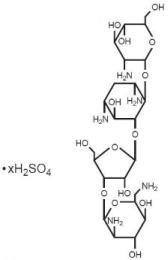
Each capsule, for oral administration, contains paromomycin sulfate equivalent to 250 mg paromomycin. Each capsule also contains the following inactive ingredients: FD&C Blue # 1, D&C Red # 28, FD&C Red # 40, gelatin and titanium dioxide. The imprinting ink for the 250 mg capsule contains D&C yellow #10, FD&C blue # 1, FD&C blue # 2, FD&C red # 40, iron oxide black, pharmaceutical shellac glaze, and propylene glycol.
- CLINICAL PHARMACOLOGY
-
INDICATIONS AND USAGE
Paromomycin sulfate is indicated for intestinal amebiasis–acute and chronic (NOTE-It is not effective in extraintestinal amebiasis); management of hepatic coma–as adjunctive therapy.
To reduce the development of drug-resistant bacteria and maintain the effectiveness of HUMATIN™ Capsules and other antibacterial drugs, HUMATIN™ Capsules should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
- CONTRAINDICATIONS
-
PRECAUTIONS
Prescribing HUMATIN™ Capsules in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
The use of this antibiotic, as with other antibiotics, may result in an overgrowth of nonsusceptible organisms, including fungi. Constant observation of the patient is essential. If new infections caused by nonsusceptible organisms appear during therapy, appropriate measures should be taken. The drug should be used with caution in individuals with ulcerative lesions of the bowel to avoid renal toxicity through inadvertent absorption.
Information for Patients
Patients should be counseled that antibacterial drugs including HUMATIN™ Capsules should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When HUMATIN™ Capsules is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by HUMATIN™ Capsules or other antibacterial drugs in the future.
PEDIATRIC USE
See DOSAGE AND ADMINISTRATION section.
- ADVERSE REACTIONS
- DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
-
STORAGE
Store at 20°-25°C (68°-77°F) [See USP controlled Room Temperature] Protect from moisture.
Preserve in tight containers as defined in the USP.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
MANUFACTURED FOR:
Waylis Therapeutics LLC
Wixom, MI 48393

Revised: 01/2021
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
HUMATIN
paromomycin sulfate capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:80725-250 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PAROMOMYCIN SULFATE (UNII: 845NU6GJPS) (PAROMOMYCIN - UNII:61JJC8N5ZK) PAROMOMYCIN SULFATE 250 mg Inactive Ingredients Ingredient Name Strength FD&C BLUE NO. 1 (UNII: H3R47K3TBD) D&C RED NO. 28 (UNII: 767IP0Y5NH) FD&C RED NO. 40 (UNII: WZB9127XOA) GELATIN (UNII: 2G86QN327L) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) FERROSOFERRIC OXIDE (UNII: XM0M87F357) SHELLAC (UNII: 46N107B71O) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) Product Characteristics Color WHITE (White opaque) , BLUE (Dark blue opaque) Score no score Shape CAPSULE Size 19mm Flavor Imprint Code HP;38 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:80725-250-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 04/08/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA065173 04/08/2021 Labeler - Waylis Therapeutics LLC (117678921) Registrant - Heritage Pharmaceuticals Inc. d/b/a Avet Pharmaceuticals Inc. (780779901) Establishment Name Address ID/FEI Business Operations Heritage Pharma Labs Inc. d/b/a Avet Pharmaceuticals Labs Inc. 189630168 ANALYSIS(80725-250) , LABEL(80725-250) , MANUFACTURE(80725-250) , PACK(80725-250)


