Label: NATURAL ADULT FLUORIDE- sodium monofluorophosphate paste
- NDC Code(s): 30142-223-39
- Packager: Kroger
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 12, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- WARNINGS
-
Directions
adults and children 2 years of age and older: brush teeth thoroughly, preferably after each meal or at least twice per day, or as directed by a dentist or doctor. * instruct children under 6 years of age in good brushing and rinsing habits (to minimize swallowing) * supervise children as necessary until capable of using without supervision * children under 2 years of age: Consult a dentist or doctor.
- INACTIVE INGREDIENT
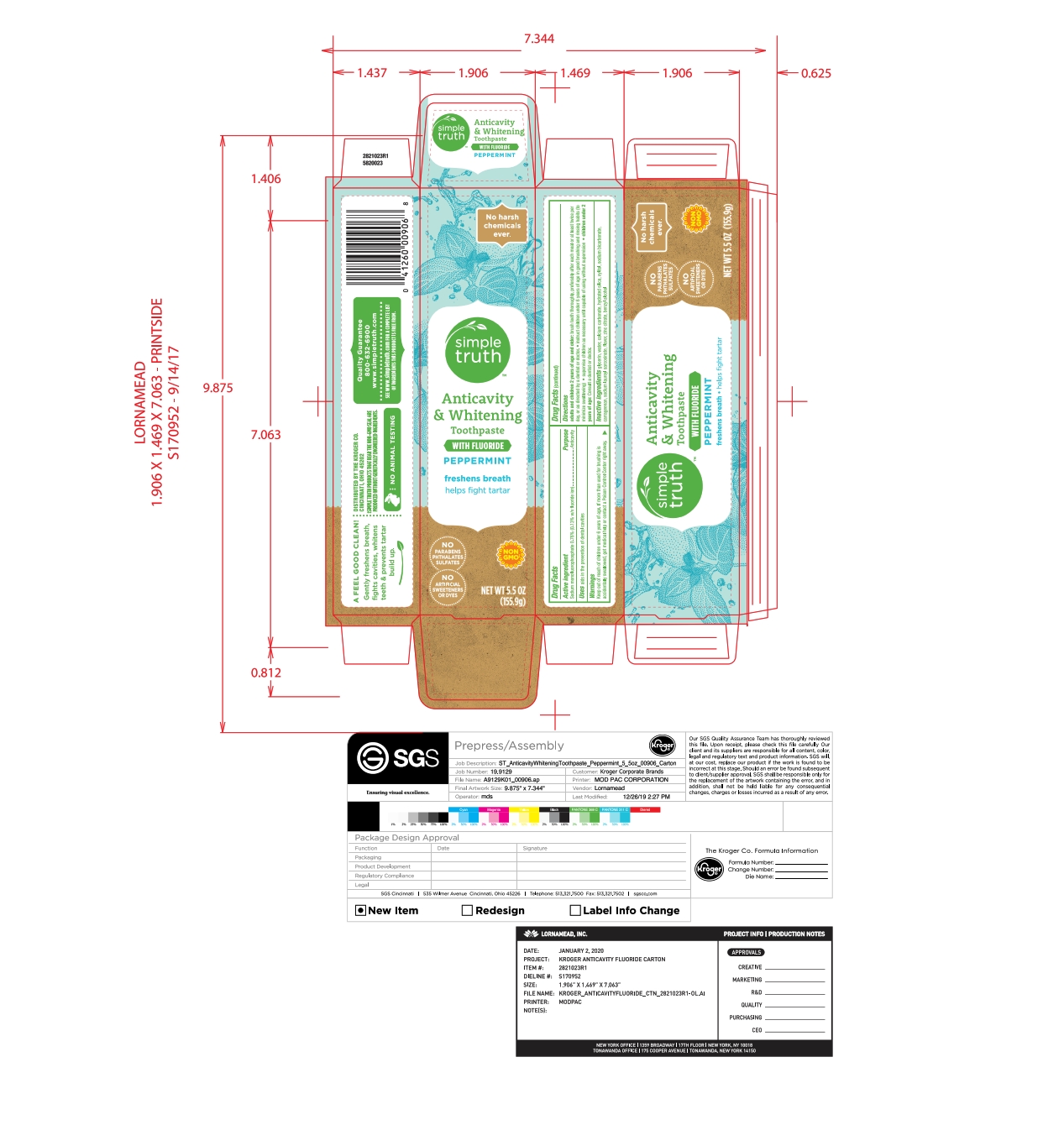
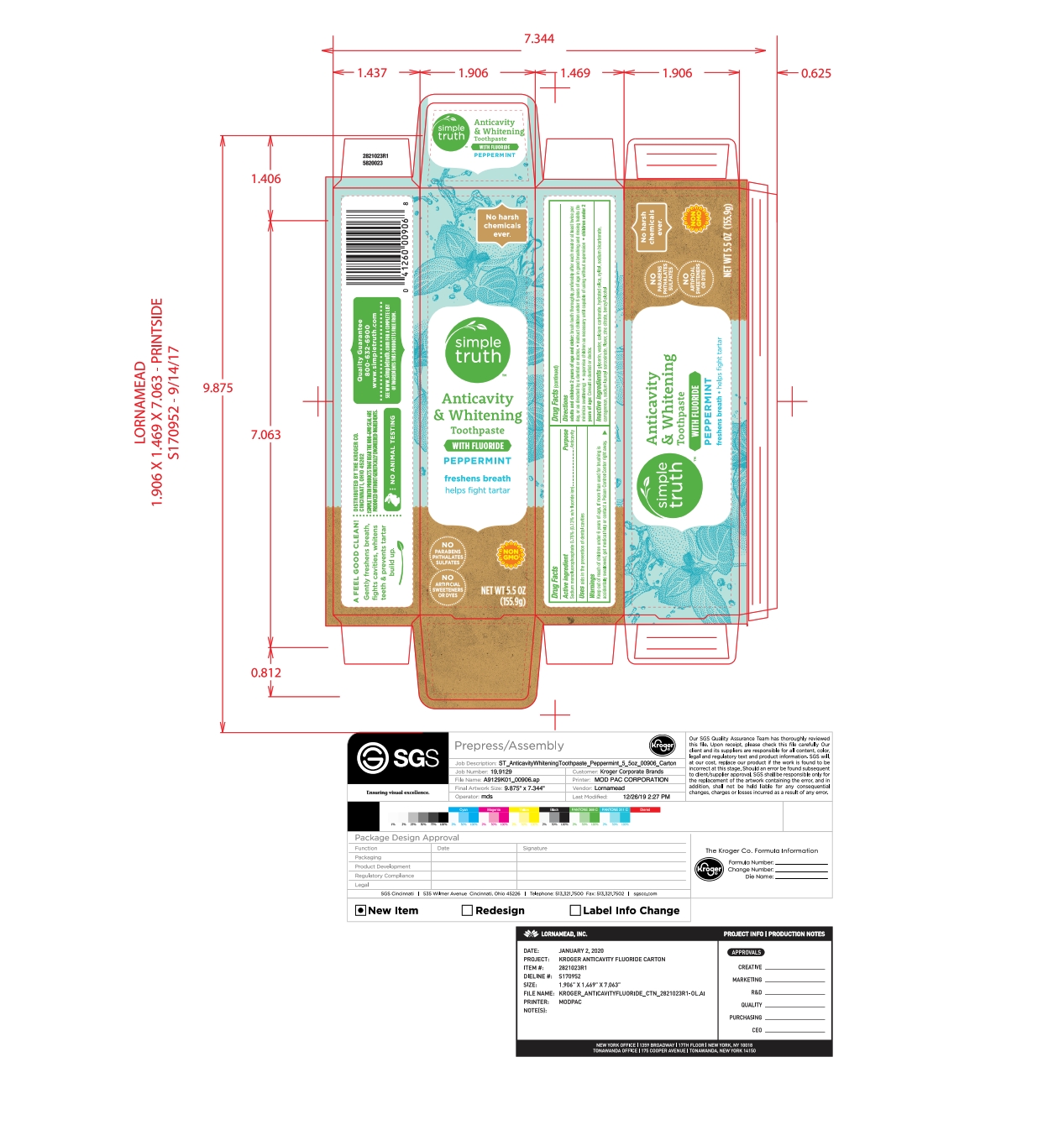
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NATURAL ADULT FLUORIDE
sodium monofluorophosphate pasteProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:30142-223 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM MONOFLUOROPHOSPHATE (UNII: C810JCZ56Q) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 0.76 g in 100 g Inactive Ingredients Ingredient Name Strength CARRAGEENAN (UNII: 5C69YCD2YJ) GLYCERIN (UNII: PDC6A3C0OX) SODIUM LAUROYL SARCOSINATE (UNII: 632GS99618) WATER (UNII: 059QF0KO0R) XYLITOL (UNII: VCQ006KQ1E) ZINC CITRATE (UNII: K72I3DEX9B) SODIUM BICARBONATE (UNII: 8MDF5V39QO) CALCIUM CARBONATE (UNII: H0G9379FGK) HYDRATED SILICA (UNII: Y6O7T4G8P9) BENZYL ALCOHOL (UNII: LKG8494WBH) Product Characteristics Color white (Off white) Score Shape Size Flavor MINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:30142-223-39 1 in 1 CARTON 06/02/2020 1 155.9 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part355 06/02/2020 Labeler - Kroger (006999528) Registrant - Lornamead (126440440) Establishment Name Address ID/FEI Business Operations Lornamead 080046418 manufacture(30142-223)