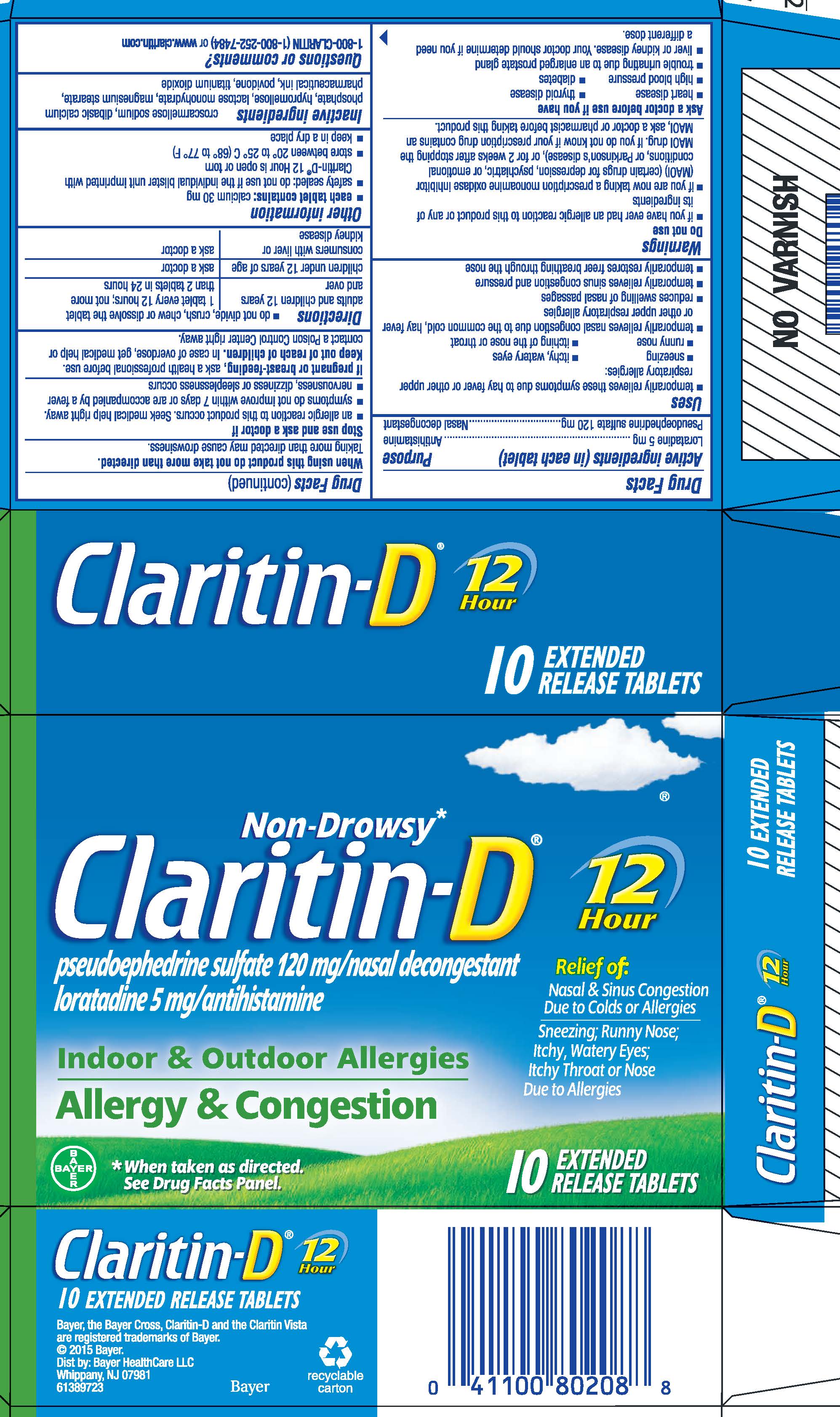
Label: CLARITIN-D 12 HOUR- loratadine and pseudoephedrine sulfate tablet, extended release
- NDC Code(s): 11523-7162-1, 11523-7162-2, 11523-7162-3
- Packager: Bayer HealthCare LLC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 5, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
-
Uses
- temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- sneezing
- itchy, watery eyes
- runny nose
- itching of the nose or throat
- temporarily relieves nasal congestion due to the common cold, hay fever or other upper respiratory allergies
- reduces swelling of nasal passages
- temporarily relieves sinus congestion and pressure
- temporarily restores freer breathing through the nose
- temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
-
Warnings
Do not use
- if you have ever had an allergic reaction to this product or any of its ingredients
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- heart disease
- thyroid disease
- high blood pressure
- diabetes
- trouble urinating due to an enlarged prostate gland
- liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product do not take more than directed.
Taking more than directed may cause drowsiness.
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
PRINCIPAL DISPLAY PANEL - Carton
NDC 11523-7162-1
Non-Drowsy*
Claritin-D®
pseudoephedrine sulfate 120 mg/nasal decongestant
loratadine 5 mg/antihistamineIndoor & Outdoor Allergies
Allergy & Congestion12
HourRelief of:
Nasal & Sinus Congestion
Due to Colds or Allergies
Sneezing; Runny Nose;
Itchy, Watery Eyes;
Itchy Throat or Nose
Due to Allergies* When taken as directed. See Drug Facts Panel.
10
EXTENDED
RELEASE TABLETS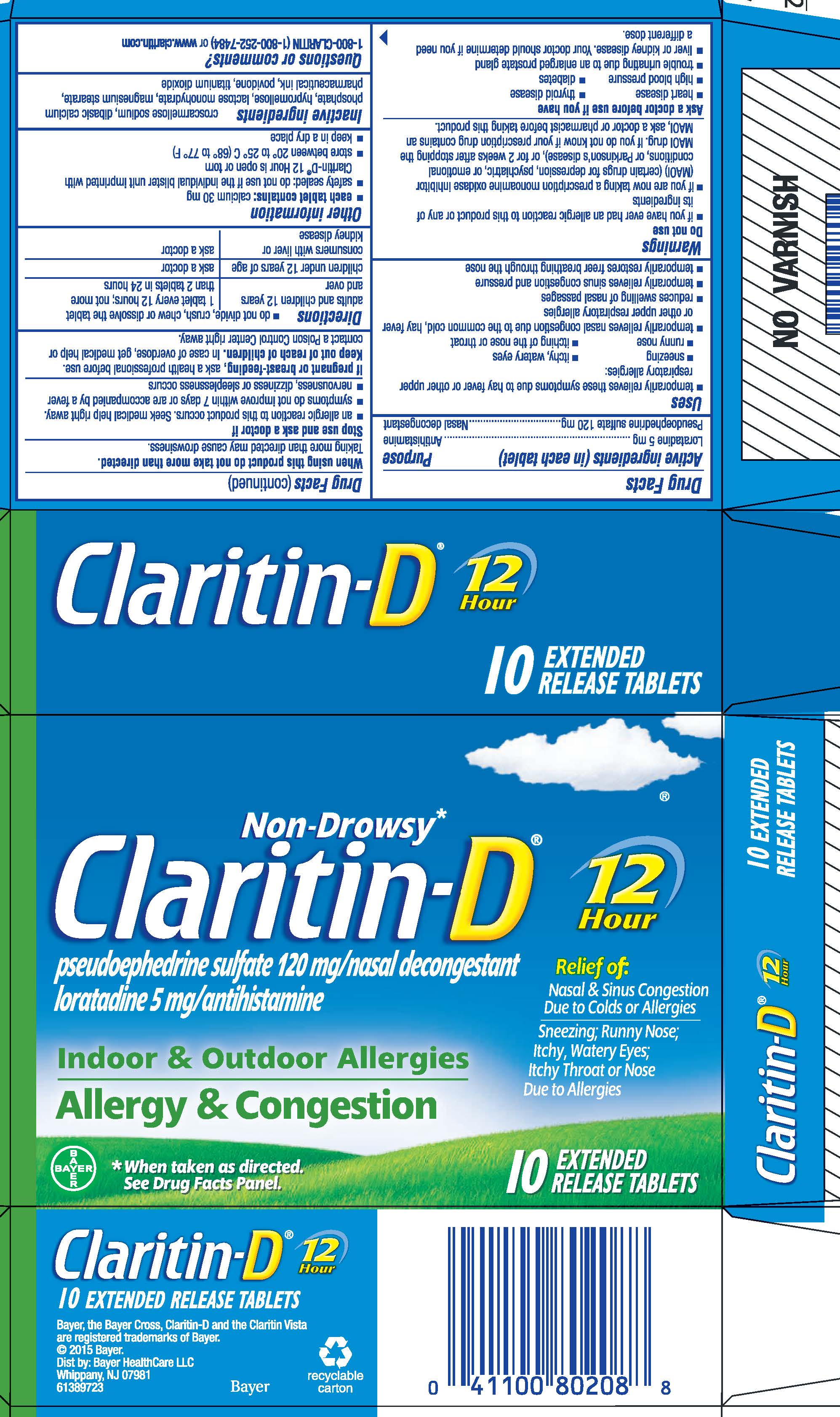
-
INGREDIENTS AND APPEARANCE
CLARITIN-D 12 HOUR
loratadine and pseudoephedrine sulfate tablet, extended releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11523-7162 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LORATADINE (UNII: 7AJO3BO7QN) (LORATADINE - UNII:7AJO3BO7QN) LORATADINE 5 mg PSEUDOEPHEDRINE SULFATE (UNII: Y9DL7QPE6B) (PSEUDOEPHEDRINE - UNII:7CUC9DDI9F) PSEUDOEPHEDRINE SULFATE 120 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) CALCIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: L11K75P92J) HYPROMELLOSES (UNII: 3NXW29V3WO) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) POVIDONE (UNII: FZ989GH94E) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color white (white to off-white) Score no score Shape OVAL Size 12mm Flavor Imprint Code Claritin;D;12 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11523-7162-1 1 in 1 CARTON 12/01/2009 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:11523-7162-2 2 in 1 CARTON 12/01/2009 2 10 in 1 BLISTER PACK; Type 0: Not a Combination Product 3 NDC:11523-7162-3 3 in 1 CARTON 12/01/2009 3 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA019670 12/01/2009 Labeler - Bayer HealthCare LLC. (112117283)