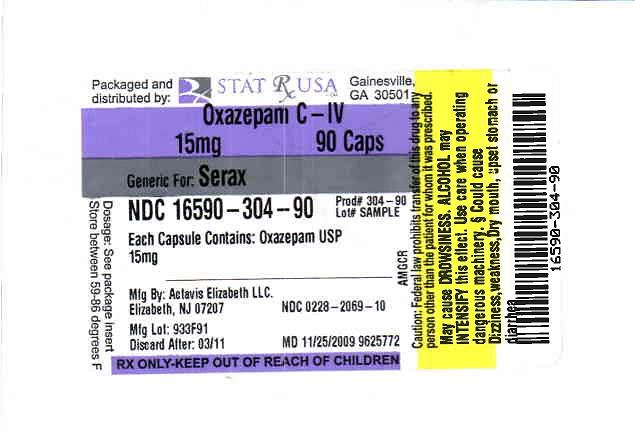
Label: OXAZEPAM capsule
-
Contains inactivated NDC Code(s)
NDC Code(s): 16590-304-30, 16590-304-60, 16590-304-90 - Packager: STAT RX USA LLC
- This is a repackaged label.
- Source NDC Code(s): 0228-2069
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CIV
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 15, 2009
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
DESCRIPTION
Oxazepam is the first of a chemical series of compounds known as the 3-hydroxybenzodiazepinones. A therapeutic agent providing versatility and flexibility in control of common emotional disturbances, this product exerts prompt action in a wide variety of disorders associated with anxiety, tension, agitation, and irritability, and anxiety associated with depression. In tolerance and toxicity studies on several animal species, this product reveals significantly greater safety factors than related compounds (chlordiazepoxide and diazepam) and manifests a wide separation of effective doses and doses inducing side effects.
Oxazepam capsules contain 10 mg, 15 mg or 30 mg oxazepam. The following inactive ingredients are contained in these capsules: corn starch, croscarmellose sodium, FDANDC Red #40, gelatin, hypromellose, lactose (monohydrate), magnesium stearate, methylparaben, propylparaben, sodium lauryl sulfate, titanium dioxide, and other inert ingredients. The 10 mg capsule also contains DANDC Red #28. The 15 mg capsule also contains DANDC Yellow #10. The 30 mg capsule also contains DANDC Red #28 and FDANDC Blue #1.
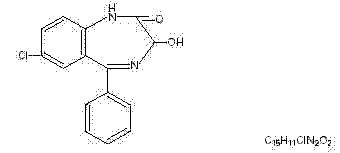
Oxazepam is 7-chloro-1,3-dihydro-3-hydroxy-5-phenyl-2H-1,4-benzodiazepin-2-one. A white crystalline powder with a molecular weight of 286.72, its structural formula is as follows:
-
CLINICAL PHARMACOLOGY
CLINICAL PHARMACOLOGY
Pharmacokinetic testing in healthy adult subjects has demonstrated that a single 30 mg dose of a capsule or tablet will result in equivalent extent of absorption. Peak plasma levels were observed to occur about 3 hours after dosing. The mean elimination half-life for oxazepam was approximately 8.2 hours (range 5.7 to 10.9 hours).
This product has a single, major inactive metabolite in man, a glucuronide excreted in the urine.
Age (less than 80 years old) does not appear to have a clinically significant effect on oxazepam kinetics. A statistically significant increase in elimination half-life in the very elderly (greater than 80 years of age) as compared to younger subjects has been reported, due to a 30% increase in volume of distribution, as well as a 50% reduction in unbound clearance of oxazepam in the very elderly. (see PRECAUTIONS, Geriatric Use).
-
INDICATIONS & USAGE
INDICATIONS AND USAGE
Oxazepam is indicated for the management of anxiety disorders or for the short-term relief of the symptoms of anxiety. Anxiety or tension associated with the stress of everyday life usually does not require treatment with an anxiolytic.
Anxiety associated with depression is also responsive to Oxazepam therapy.
This product has been found particularly useful in the management of anxiety, tension, agitation, and irritability in older patients.
Alcoholics with acute tremulousness, inebriation, or with anxiety, associated with alcohol withdrawal are responsive to therapy.
The effectiveness of Oxazepam in long-term use, that is, more than 4 months, has not been assessed by systematic clinical studies. The physician should periodically reassess the usefulness of the drug for the individual patient.
- CONTRAINDICATIONS
-
WARNINGS AND PRECAUTIONS
WARNINGS
As with other CNS-acting drugs, patients should be cautioned against driving automobiles or operating dangerous machinery until it is known that they do not become drowsy or dizzy on oxazepam therapy.
Patients should be warned that the effects of alcohol or other CNS-depressant drugs may be additive to those of Oxazepam, possibly requiring adjustment of dosage or elimination of such agents.
Withdrawal symptoms of the barbiturate type have occurred after the discontinuation of benzodiazepines (see DRUG ABUSE AND DEPENDENCE section).
Use in Pregnancy:An increased risk of congenital malformations associated with the use of minor tranquilizers (chlordiazepoxide, diazepam, and meprobamate) during the first trimester of pregnancy has been suggested in several studies. Oxazepam, a benzodiazepine derivative, has not been studied adequately to determine whether it, too, may be associated with an increased risk of fetal abnormality. Because use of these drugs is rarely a matter of urgency, their use during this period should almost always be avoided. The possibility that a woman of childbearing potential may be pregnant at the time of institution of therapy should be considered. Patients should be advised that if they become pregnant during therapy or intend to become pregnant they should communicate with their physician about the desirability of discontinuing the drug.
PRECAUTIONSGeneralAlthough hypotension has occurred only rarely, oxazepam should be administered with caution to patients in whom a drop in blood pressure might lead to cardiac complications. This is particularly true in the elderly patient.
Information for PatientsTo assure the safe and effective use of oxazepam, patients should be informed that, since benzodiazepines may produce psychological and physical dependence, it is advisable that they consult with their physician before either increasing the dose or abruptly discontinuing this drug.
Pediatric UseSafety and effectiveness in pediatric patients under 6 years of age have not been established. Absolute dosage for pediatric patients 6 to 12 years of age is not established.
Geriatric UseClinical studies of oxazepam were not adequate to determine whether subjects aged 65 and over respond differently than younger subjects. Age (less than 80 years old) does not appear to have a clinically significant effect on oxazepam kinetics (see CLINICAL PHARMACOLOGY).
Clinical circumstances, some of which may be more common in the elderly, such as hepatic or renal impairment, should be considered. Greater sensitivity of some older individuals to the effects of oxazepam (e.g., sedation, hypotension, paradoxical excitation) cannot be ruled out (see PRECAUTIONS, General; see ADVERSE REACTIONS). In general, dose selection for oxazepam for elderly patients should be cautious, usually starting at the lower end of the dosing range (see DOSAGE AND ADMINISTRATION).
-
ADVERSE REACTIONS
ADVERSE REACTIONS
The necessity for discontinuation of therapy due to undesirable effects has been rare. Transient, mild drowsiness is commonly seen in the first few days of therapy. If it persists, the dosage should be reduced. In few instances, dizziness, vertigo, headache, and rarely syncope have occurred either alone or together with drowsiness. Mild paradoxical reactions, i.e., excitement, stimulation of affect, have been reported in psychiatric patients; these reactions may be secondary to relief of anxiety and usually appear in the first two weeks of therapy.
Other side effects occurring during oxazepam therapy include rare instances of nausea, lethargy, edema, slurred speech, tremor, altered libido, and minor diffuse skin rashes — morbilliform, urticarial, and maculopapular. Such side effects have been infrequent and are generally controlled with reduction of dosage. A case of an extensive fixed drug eruption also has been reported.
Although rare, leukopenia and hepatic dysfunction including jaundice have been reported during therapy. Periodic blood counts and liver-function tests are advisable.
Ataxia with oxazepam has been reported in rare instances and does not appear to be specifically related to dose or age.
Although the following side reactions have not as yet been reported with oxazepam, they have occurred with related compounds (chlordiazepoxide and diazepam): paradoxical excitation with severe rage reactions, hallucinations, menstrual irregularities, change in EEG pattern, blood dyscrasias including agranulocytosis, blurred vision, diplopia, incontinence, stupor, disorientation, fever, and euphoria.
Transient amnesia or memory impairment has been reported in association with the use of benzodiazepines
-
DRUG ABUSE AND DEPENDENCE
DRUG ABUSE AND DEPENDENCE
Oxazepam Capsules are classified by the Drug Enforcement Administration as a schedule IV controlled substance.
Withdrawal symptoms, similar in character to those noted with barbiturates and alcohol (convulsions, tremor, abdominal and muscle cramps, vomiting, and sweating), have occurred following abrupt discontinuance of oxazepam. The more severe withdrawal symptoms have usually been limited to those patients who received excessive doses over an extended period of time. Generally milder withdrawal symptoms (e.g., dysphoria and insomnia) have been reported following abrupt discontinuance of benzodiazepines taken continuously at therapeutic levels for several months. Consequently, after extended therapy, abrupt discontinuation should generally be avoided and a gradual dosage-tapering schedule followed. Addiction-prone individuals (such as drug addicts or alcoholics) should be under careful surveillance when receiving oxazepam or other psychotropic agents because of the predisposition of such patients to habituation and dependence.
-
OVERDOSAGE
OVERDOSAGE
In the management of overdosage with any drug, it should be borne in mind that multiple agents may have been taken.
Symptoms:Overdosage of benzodiazepines is usually manifested by varying degrees of central nervous system depression ranging from drowsiness to coma. In mild cases, symptoms include drowsiness, mental confusion and lethargy. In more serious cases, and especially when other drugs or alcohol were ingested, symptoms may include ataxia, hypotonia, hypotension, hypnotic state, stage one (1) to three (3) coma, and very rarely, death.
Management:Induced vomiting and/or gastric lavage should be undertaken, followed by general supportive care, monitoring of vital signs, and close observation of the patient. Hypotension, though unlikely, usually may be controlled with norepinephrine bitartrate injection. The value of dialysis has not been adequately determined for oxazepam.
The benzodiazepine antagonist flumazenil may be used in hospitalized patients as an adjunct to, not as a substitute for, proper management of benzodiazepine overdose. The prescriber should be aware of a risk of seizure in association with flumazenil treatment, particularly in long-term benzodiazepine users and in cyclic antidepressant overdose. The complete flumazenil package insert including CONTRAINDICATIONS, WARNINGS, and PRECAUTIONS should be consulted prior to use.
-
DOSAGE & ADMINISTRATION
DOSAGE AND ADMINISTRATION
Because of the flexibility of this product and the range of emotional disturbances responsive to it, dosage should be individualized for maximum beneficial effects.
USUAL DOSE
Mild-to-moderate anxiety, with associated tension, irritability, agitation, or related symptoms of functional origin or secondary to organic disease. 10 to 15 mg, 3 or 4 times daily Severe anxiety syndromes, agitation, or anxiety associated with depression. 15 to 30 mg, 3 or 4 times daily Older patients with anxiety, tension, irritability, and agitation. Initial dosage: 10 mg, 3 times daily. If necessary, increase cautiously to 15 mg, 3 or 4 times daily. Alcoholics with acute inebriation, tremulousness, or anxiety on withdrawal. 15 to 30 mg, 3 or 4 times daily This product is not indicated in pediatric patients under 6 years of age. Absolute dosage for pediatric patients 6 to 12 years of age is not established.
-
HOW SUPPLIED
HOW SUPPLIED
Oxazepam capsules are available as follows:
10 mg — Each pink opaque gelatin #4 capsule printed with Image from Drug Label Content and 067 in black ink on both cap and body contains 10 mg of Oxazepam, USP. Capsules are supplied in bottles of 100 (NDC 0228-2067-10) AND 500 (NDC 0228-2067-50).
15 mg — Each red opaque gelatin #4 capsule printed with Image from Drug Label Content and 069 in black ink on both cap and body contains 15 mg of Oxazepam, USP. Capsules are supplied in bottles of 100 (NDC 0228-2069-10) and 500 (NDC 0228-2069-50).
30 mg — Each maroon opaque gelatin #4 capsule printed with Image from Drug Label Content and 073 in blue ink on both cap and body contains 30 mg of Oxazepam, USP. Capsules are supplied in bottles of 100 (NDC 0228-2073-10).
Keep tightly closed.
Dispense in a tight, light-resistant container as defined in the USP.
Store at controlled room temperature 15°-30°C (59°-86°F).
Rx only
-
ANIMAL PHARMACOLOGY & OR TOXICOLOGY
ANIMAL PHARMACOLOGY AND/OR ANIMAL TOXICOLOGY
In mice, oxazepam exerts an anticonvulsant (anti-Metrazol®) activity at 50-percent-effective doses of about 0.6 mg/kg orally. (Such anticonvulsant activity of benzodiazepines correlates with their tranquilizing properties.) To produce ataxia (rotabar test) and sedation (abolition of spontaneous motor activity), the 50-percent-effective doses of this product are greater than 5 mg/kg orally. Thus, about ten times the therapeutic (anticonvulsant) dose must be given before ataxia ensues, indicating a wide separation of effective doses and doses inducing side effects.
In evaluation of antianxiety of compounds, conflict behavioral tests in rats differentiate continuous response for food in the presence of anxiety-provoking stress (shock) from drug-induced motor incoordination. This product shows significant separation of doses required to relieve anxiety and doses producing sedation or ataxia. Ataxia-producing doses exceed those of related CNS-acting drugs.
Acute oral LD50 in mice is greater than 5000 mg/kg, compared to 800 mg/kg for a related compound (chlordiazepoxide).
Subacute toxicity studies in dogs for four weeks at 480 mg/kg daily showed no specific changes; at 960 mg/kg two out of eight died with evidence of circulatory collapse. This wide margin of safety is significant compared to chlordiazepoxide HCI, which showed nonspecific changes in six dogs at 80 mg/kg. On chlordiazepoxide, two out of six died with evidence of circulatory collapse at 127 mg/kg, and six out of six died at 200 mg/kg daily. Chronic toxicity studies of oxazepam in dogs at 120 mg/kg/day for 52 weeks produced no toxic manifestation.
Fatty metamorphosis of the liver has been noted in six-week toxicity studies in rats given this product at 0.5% of the diet. Such accumulations of fat are considered reversible, as there is no liver necrosis or fibrosis.
Breeding studies in rats through two successive litters did not produce fetal abnormality.
Oxazepam has not been adequately evaluated for mutagenic activity.
In a carcinogenicity study, oxazepam was administered with diet to rats for two years. Male rats receiving 30 times the maximum human dose showed a statistical increase, when compared to controls, in benign thyroid follicular cell tumors, testicular interstitial cell adenomas, and prostatic adenomas. An earlier published study reported that mice fed dietary dosages of 35 or 100 times the human daily dose of oxazepam for 9 months developed a dose-related increase in liver adenomas.1 In an independent analysis of some of the microscopic slides from this mouse study, several of these tumors were classified as liver carcinomas. At this time, there is no evidence that clinical use of oxazepam is associated with tumors
- REFERENCES
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
OXAZEPAM
oxazepam capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:16590-304(NDC:0228-2069) Route of Administration ORAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OXAZEPAM (UNII: 6GOW6DWN2A) (OXAZEPAM - UNII:6GOW6DWN2A) OXAZEPAM 15 mg Product Characteristics Color red (RED (OPAQUE)) Score 2 pieces Shape CAPSULE (CAPSULE (GELATIN)) Size 14mm Flavor Imprint Code R;069 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:16590-304-90 90 in 1 BOTTLE, PLASTIC 2 NDC:16590-304-30 30 in 1 BOTTLE, PLASTIC 3 NDC:16590-304-60 60 in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA072252 04/14/1988 Labeler - STAT RX USA LLC (786036330)