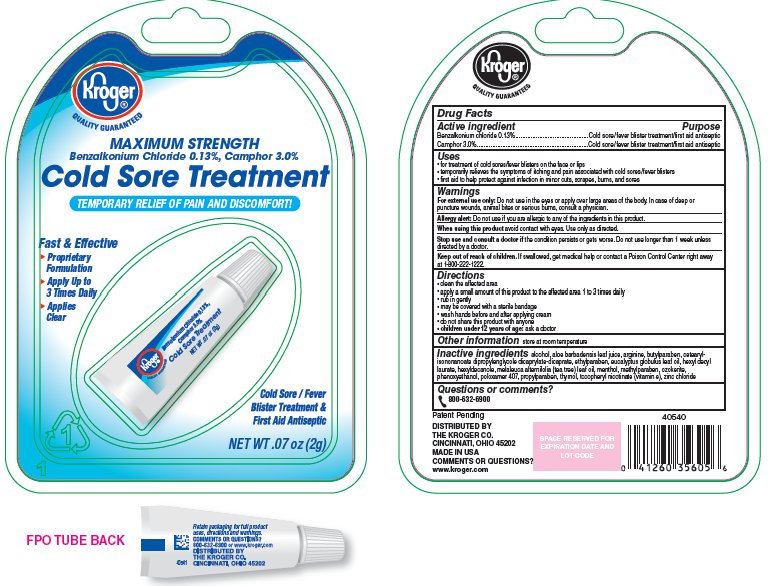
Label: KROGER COLD SORE TREATMENT- camphor cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 30142-408-01 - Packager: The Kroger Co.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 30, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Uses
-
Warnings
- For external use only: Do not use in the eyes or apply over large areas of the body. In case of deep or puncture wounds, animal bites or serious burns, consult a physician.
- Allergy Alert: Do not use if you are allergic to any of the ingredients in this product.
- When using this product avoid contact with eyes. Use only as directed.
- Stop use and consult a doctor if the condition persists or gets worse. Do not use longer than 1 week unless directed by a doctor.
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other information
-
Inactive ingredients
alcohol, aloe barbadensis leaf juice, arginine, butylparaben, cetearyl isononanoate, dipropylene glycol dicaprylate/dicaprate, ethylparaben, eucalyptus globulus leaf oil, hexyl decyl laurate, hexyldecanole, melaleuca alternifolia (tea tree) leaf oil, menthol, methylparaben, ozokerite, phenoxyethanol, poloxamer 407, propylparaben, thymol, tocopheryl nicotinate (vitamin e), zinc chloride
- Questions or Comments?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
KROGER COLD SORE TREATMENT
camphor creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:30142-408 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Benzalkonium Chloride (UNII: F5UM2KM3W7) (Benzalkonium - UNII:7N6JUD5X6Y) Benzalkonium Chloride 1.3 mg in 1 g CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) (CAMPHOR (SYNTHETIC) - UNII:5TJD82A1ET) CAMPHOR (SYNTHETIC) 30 mg in 1 g Inactive Ingredients Ingredient Name Strength Alcohol (UNII: 3K9958V90M) Aloe Vera Leaf (UNII: ZY81Z83H0X) Arginine (UNII: 94ZLA3W45F) Butylparaben (UNII: 3QPI1U3FV8) Ceresin (UNII: Q1LS2UJO3A) Cetearyl Isononanoate (UNII: P5O01U99NI) Corymbia Citriodora Leaf Oil (UNII: M63U6N96EB) Dipropylene Glycol Caprate/Caprylate Diester (UNII: R6G12EY23X) Ethylparaben (UNII: 14255EXE39) Hexyldecanol (UNII: 151Z7P1317) Hexyldecyl Laurate (UNII: 0V595C1P6M) Levomenthol (UNII: BZ1R15MTK7) Methylparaben (UNII: A2I8C7HI9T) PHENOXYETHANOL (UNII: HIE492ZZ3T) Poloxamer 407 (UNII: TUF2IVW3M2) Propylparaben (UNII: Z8IX2SC1OH) Tea Tree Oil (UNII: VIF565UC2G) Thymol (UNII: 3J50XA376E) TOCOPHERYL NICOTINATE, D-.ALPHA. (UNII: WI1J5UCY5C) Zinc Chloride (UNII: 86Q357L16B) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:30142-408-01 1 in 1 PACKAGE 1 2 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 04/30/2013 Labeler - The Kroger Co. (006999528) Registrant - Ranir LLC (364567615) Establishment Name Address ID/FEI Business Operations Ranir LLC 364567615 RELABEL(30142-408) , REPACK(30142-408)