Label: TUN-L-XL 191-2540- kit
- NHRIC Code(s): 60816-2540-1
- Packager: Epimed International
- Category: MEDICAL DEVICE
- DEA Schedule: None
- Marketing Status: Premarket Notification
Drug Label Information
Updated September 30, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DESCRIPTION
-
WARNINGS
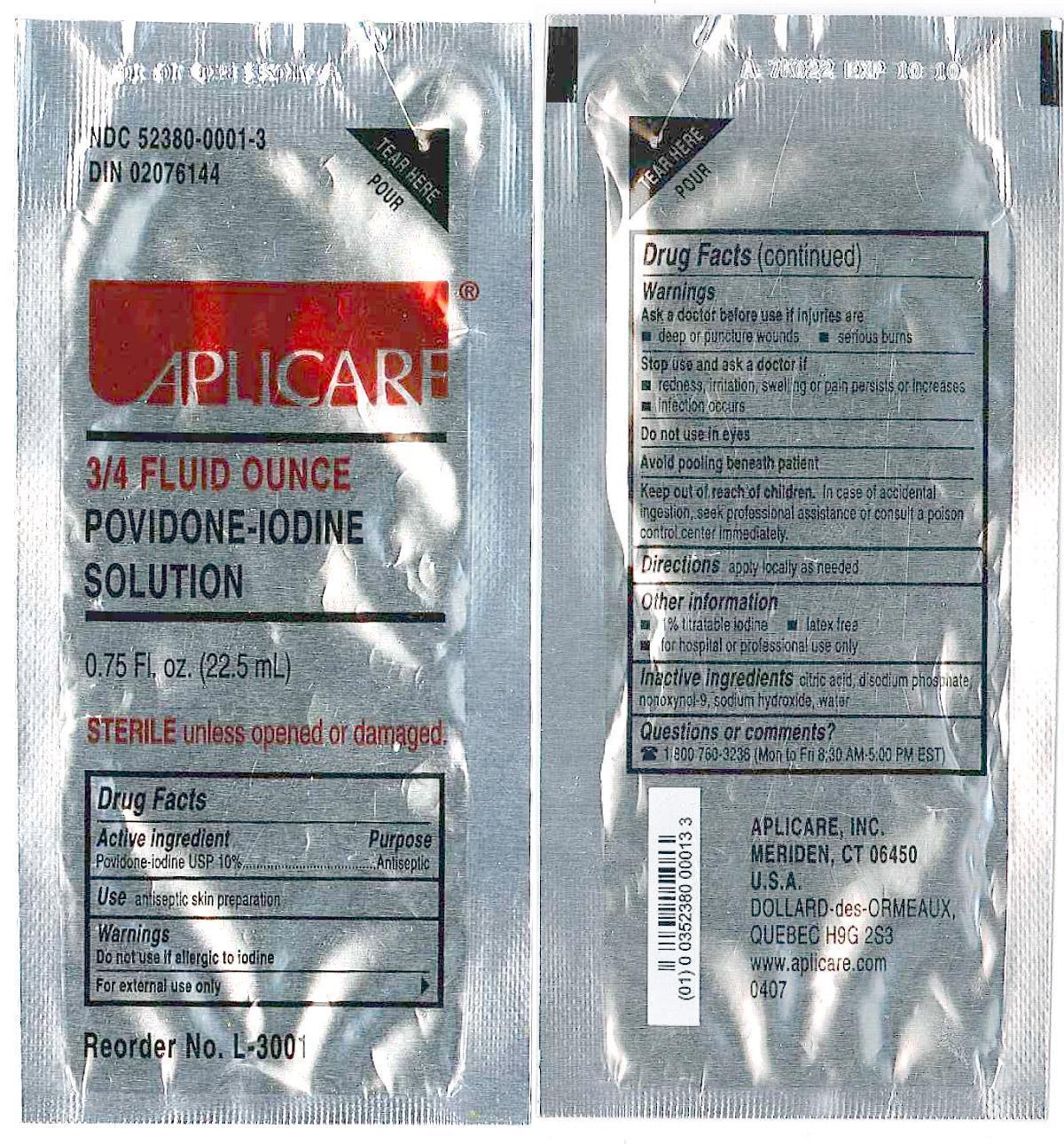
Povidone-iodine 10%
Antiseptic
Warnings
Do not use
- if allergic to iodine
- in the eyes
For external use only
Ask a doctor before use if injuries are
- deep or puncture wounds
- serious burns
Stop use and ask a doctor if
- redness, irritation, swelling or pain persists or increases
- infection occurs
Avoid pooling beneath patient
Keep out of reach of children.In case of accidental ingestion, seek professionalassistance or consult a poison control center immediately.
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
TUN-L-XL 191-2540
catheter, conduction, anesthetic kitProduct Information Product Type MEDICAL DEVICE Item Code (Source) NHRIC:60816-2540 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:60816-2540-1 1 in 1 PACKAGE Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 PACKET 22.5 mL Part 1 of 1 APLICARE POVIDONE-IODINE
povidone-iodine solutionProduct Information Item Code (Source) NDC:52380-0001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Povidone-iodine (UNII: 85H0HZU99M) (IODINE - UNII:9679TC07X4) IODINE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM PHOSPHATE, DIBASIC (UNII: GR686LBA74) SODIUM HYDROXIDE (UNII: 55X04QC32I) NONOXYNOL-9 (UNII: 48Q180SH9T) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 22.5 mL in 1 PACKET Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date premarket notification K954584 10/08/1996 Labeler - Epimed International (799751953) Establishment Name Address ID/FEI Business Operations Epimed International 799751953 relabel, manufacture Establishment Name Address ID/FEI Business Operations Aplicare, Inc. 107255002 manufacture