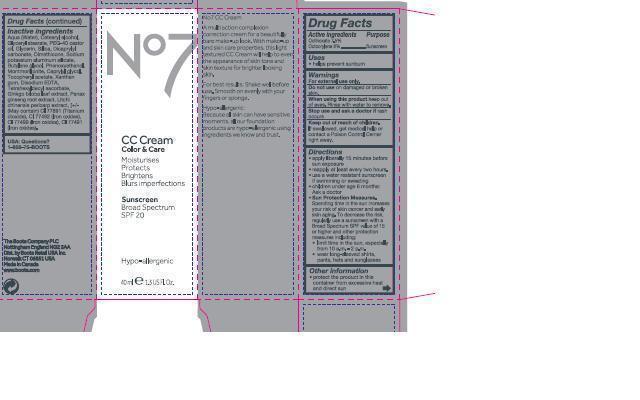
Label: NO7 CC CREAM MEDIUM- octinoxate, octocrylene emulsion
-
Contains inactivated NDC Code(s)
NDC Code(s): 68472-160-01 - Packager: Boots Retail USA Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 1, 2014
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Carton Active Ingredients Section
- Uses
- WARNINGS AND PRECAUTIONS
- Ask a doctor
- Keep out of reach of children
-
Directions
Directions
apply liberally 15 minutes before sun exposure
reapply at least every two hours
use a water resistant sunscreen if swimming or sweating
children under 6 months of age: Ask a doctor
Sun Protection Measures. spending time in the sun increases your risk of skin cancer and early skin aging. To decrease the risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other protection measures including:
limit time in the sun, especially from 10 a.m. - 2 p.m.
wear long-sleeved shirts, pants, hats and sunglasses.
- Storage
-
Inactive ingredients
Aqua(Water), Cetearyl alcohol, Glyceryl stearate, PEG-40 castor oil, Glycerin, Silica, Dicaprylyl carbonate, Dimethicone, Sodium potassium aluminum silicate, Butylene glycol, Phenoxyethanol, Montmorillonite, Caprylyl glycol, Tocopheryl acetate, Xanthan gum, Disodium EDTA, Tetrahexyldecyl ascorbate, Ginkgo biloba leaf extract, Litchi chinensis pericarp extract CI 77891 (Titanium dioxide), CI 77492 (Iron oxides) CI 77491 (Iron oxides), CI 77499 (Iron oxides)
-
Description
No7 CC Cream
A multi action complexion correction cream for a beautifully bare make up look. With make-up and skincare properties, this light textured CC Cream will help to even the appearance of skin tone and skin texture for brighter looking skin. For best results: Shake well before use. Smooth on evenly with your fingers or sponge.
- Information
- Tube
- Warnings
- Description
- Information
- CC Cream Carton
-
INGREDIENTS AND APPEARANCE
NO7 CC CREAM MEDIUM
octinoxate, octocrylene emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68472-160 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 3.2 g in 40 g OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 3 g in 40 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) PEG-40 CASTOR OIL (UNII: 4ERD2076EF) GLYCERIN (UNII: PDC6A3C0OX) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) MONTMORILLONITE (UNII: A585MN1H2L) XANTHAN GUM (UNII: TTV12P4NEE) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) GINKGO BILOBA LEAF OIL (UNII: Y5967KO1JH) PANAX GINSENG ROOT OIL (UNII: P9T4K47OM0) CAPRYLYL GLYCOL (UNII: 00YIU5438U) PHENOXYETHANOL (UNII: HIE492ZZ3T) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68472-160-01 1 in 1 CARTON 1 40 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 04/30/2014 Labeler - Boots Retail USA Inc (143151533) Registrant - The Boots Company PLC (218622660) Establishment Name Address ID/FEI Business Operations Cosmetica Laboratories Inc 255080491 manufacture(68472-160)