Label: SENNOSIDES capsule, liquid filled
- NDC Code(s): 69848-016-10
- Packager: Granules USA Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 27, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Uses
- Do not use
- Ask a doctor before use if you have
- Stop use and consult a doctor if
- If pregnant or breast-feeding
- Keep out of reach of children.
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
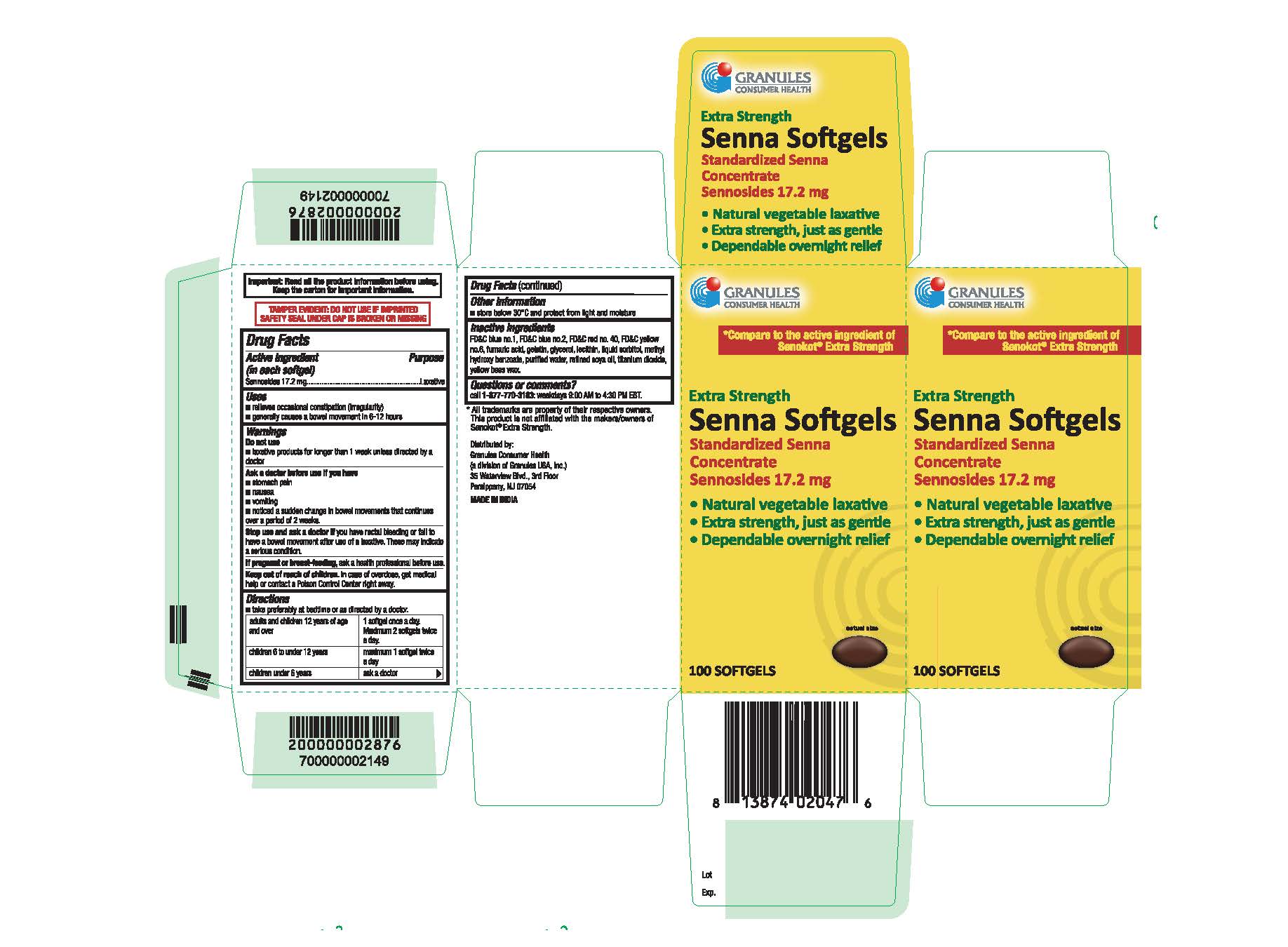
- Package Label Principal Display Panel
-
INGREDIENTS AND APPEARANCE
SENNOSIDES
sennosides capsule, liquid filledProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69848-016 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SENNOSIDES (UNII: 3FYP5M0IJX) (SENNOSIDES - UNII:3FYP5M0IJX) SENNOSIDES 17.2 mg in 17.2 Inactive Ingredients Ingredient Name Strength FD&C BLUE NO. 2 (UNII: L06K8R7DQK) FD&C RED NO. 40 (UNII: WZB9127XOA) GELATIN (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) METHYLPARABEN (UNII: A2I8C7HI9T) WATER (UNII: 059QF0KO0R) YELLOW WAX (UNII: 2ZA36H0S2V) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) SOYBEAN OIL (UNII: 241ATL177A) FUMARIC ACID (UNII: 88XHZ13131) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) SORBITOL (UNII: 506T60A25R) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) Product Characteristics Color brown (Maroon coloured, opaque soft gelatin capsules containing dark brown coloured oily mass.) Score no score Shape OVAL Size 14mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69848-016-10 100 in 1 BOTTLE; Type 0: Not a Combination Product 05/29/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M007 05/29/2020 Labeler - Granules USA Inc (137098864)