Label: INSTANT HAND SANITIZER gel
-
Contains inactivated NDC Code(s)
NDC Code(s): 72619-1001-3, 72619-1219-2, 72619-1219-3, 72619-2020-1, view more72619-2020-2, 72619-2020-4 - Packager: Acessory Myxx LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated May 19, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Instant Hand Sanitizer
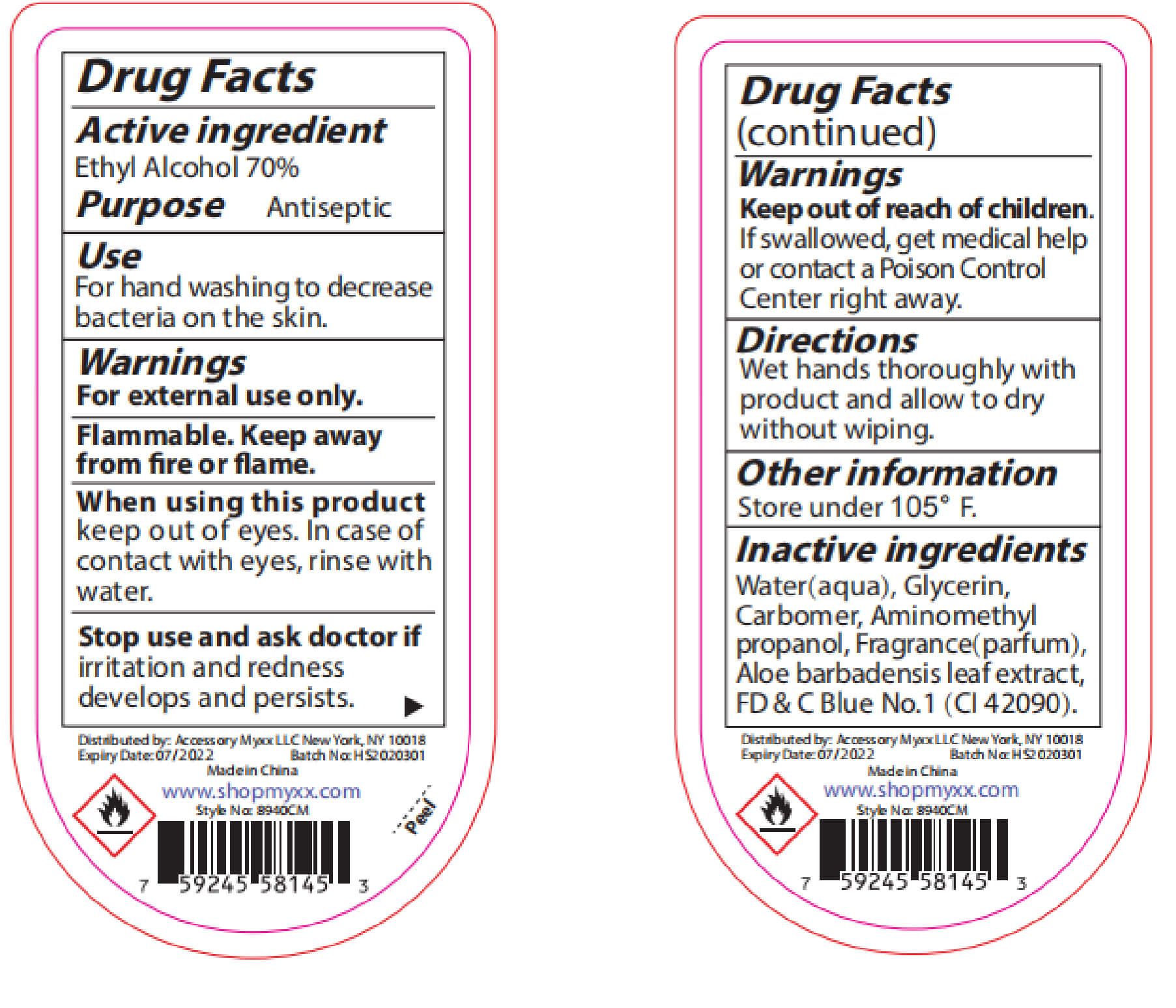
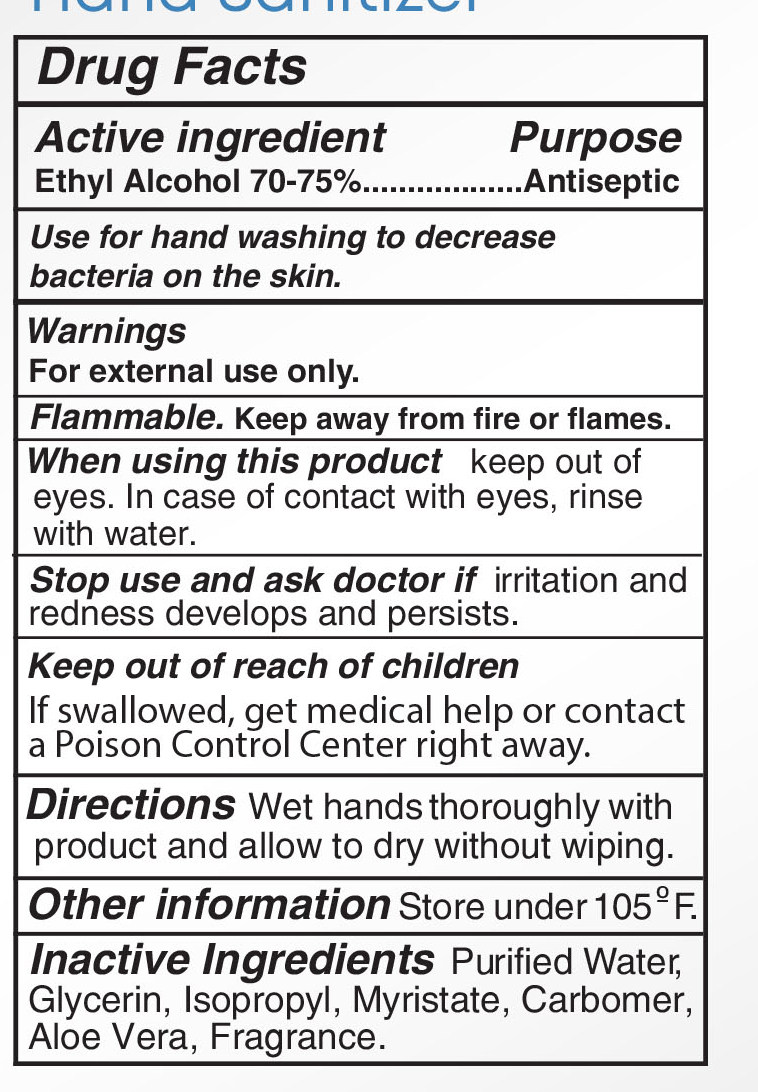
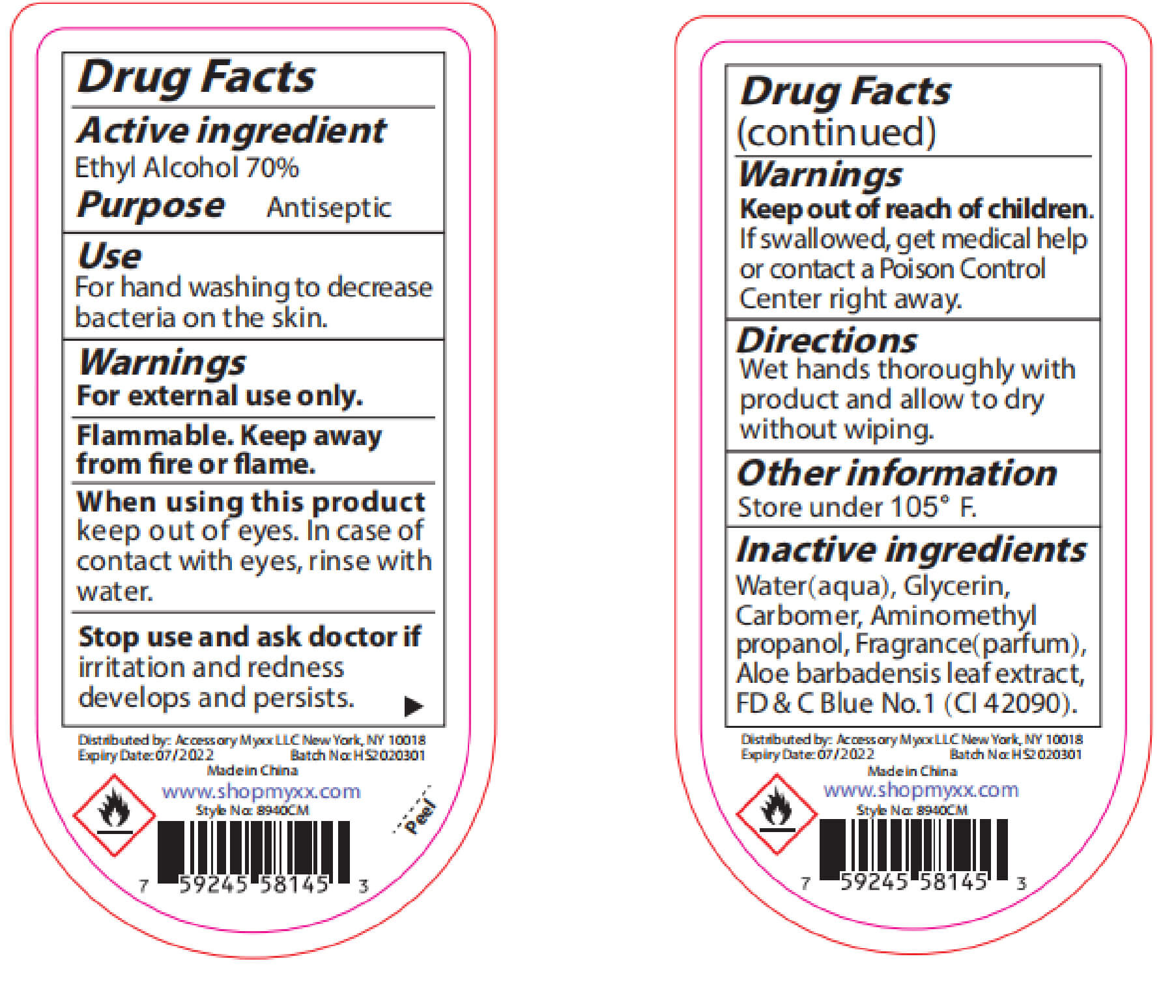
Warnings
For external use only: handsKeep out of reach of
children.If swallowed, get
medical help or contact a
Poison Control Center right
away.Stop use and ask a doctor
if irritation and redness
develop condition persists
for more than 72 hoursaviid contact with broken
skin do not inhale or
ingest -
General Warnings
Warnings
For external use only: handsKeep out of reach of
children.If swallowed, get
medical help or contact a
Poison Control Center right
away.Stop use and ask a doctor
if irritation and redness
develop condition persists
for more than 72 hoursaviid contact with broken
skin do not inhale or
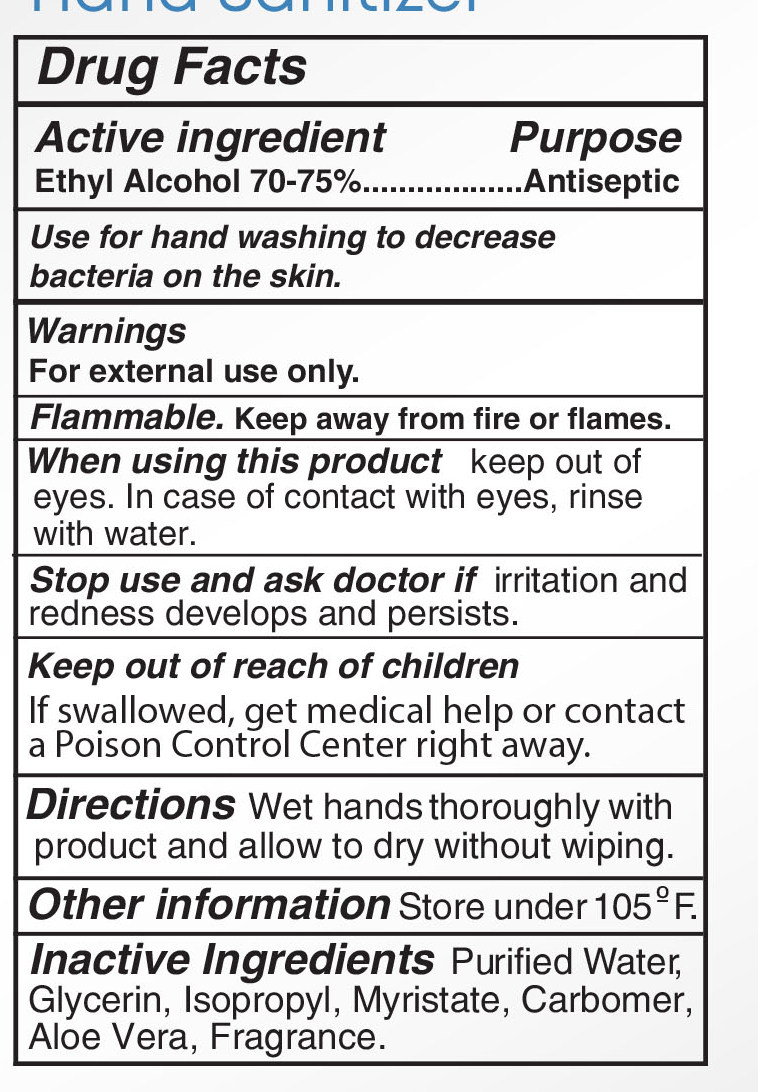
ingest - ACTIVE INGREDIENT
- INDICATIONS & USAGE
-
INACTIVE INGREDIENT
Water, Aloe Barbadensis Leaf Juice,
Maltodextrin,Glycerin, Propylene
Glycol, Acrylates/C10-30 Alkyl
Acrylate Crosspolymer,
Triethanoamine, Fragrance, Lactose,
Microcrystalline Cellulose,Sucrose,
Zea Mays(Corn) starch, Ultramarine
blue CI 77007,Tocopheryl Acetate,
Hydroxypropyl Methyl Cellulose,
D&C Red NO.33, FD&C Blue No.1 - PURPOSE
- DOSAGE & ADMINISTRATION
- KEEP OUT OF REACH OF CHILDREN
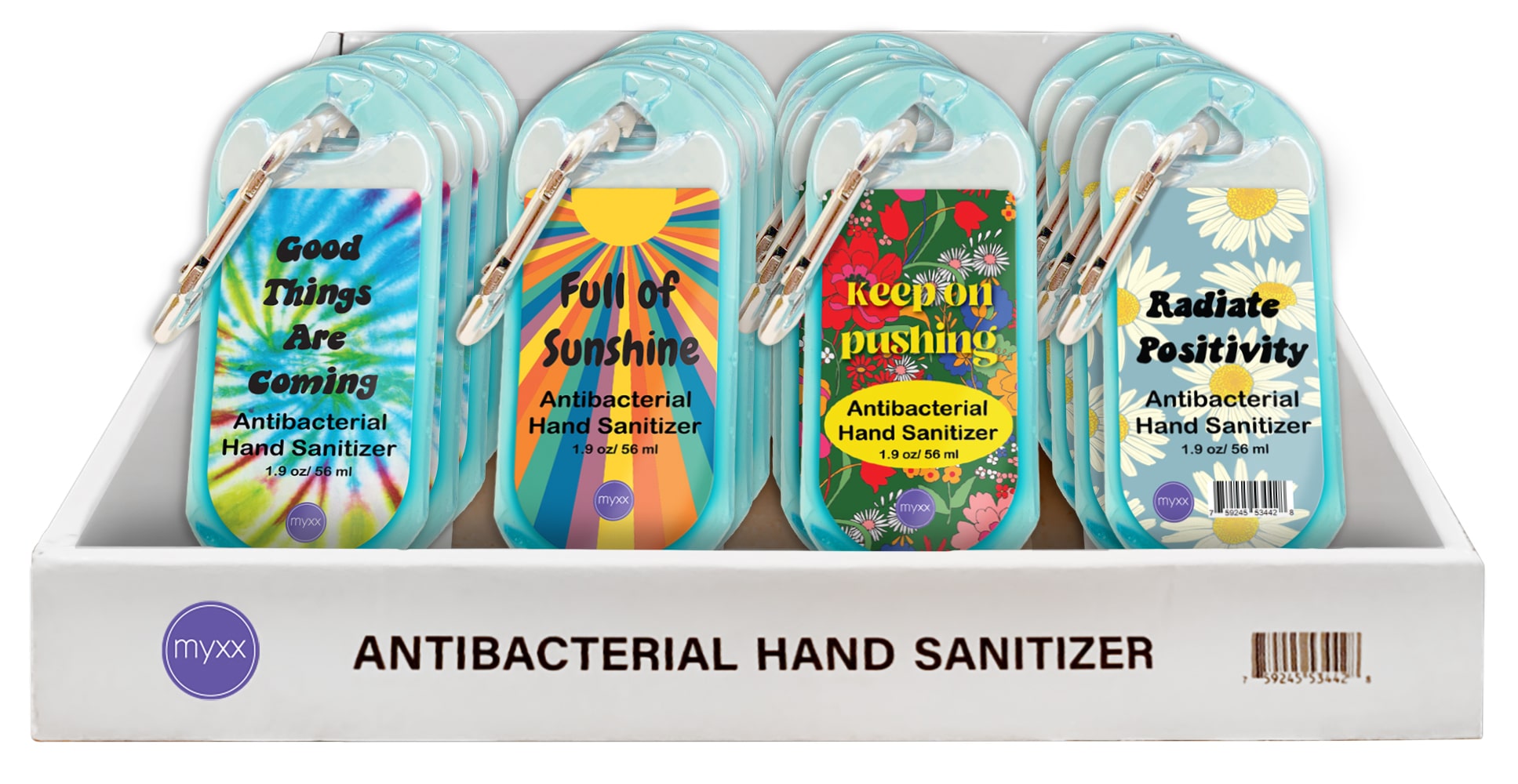
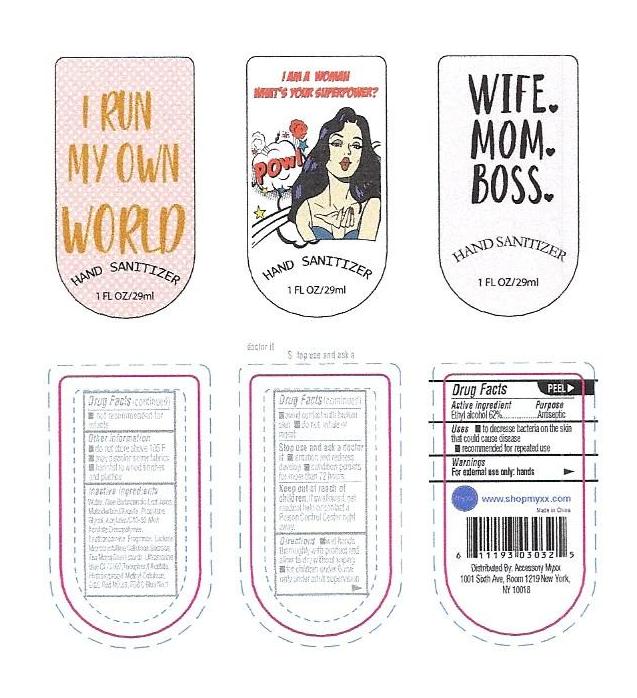
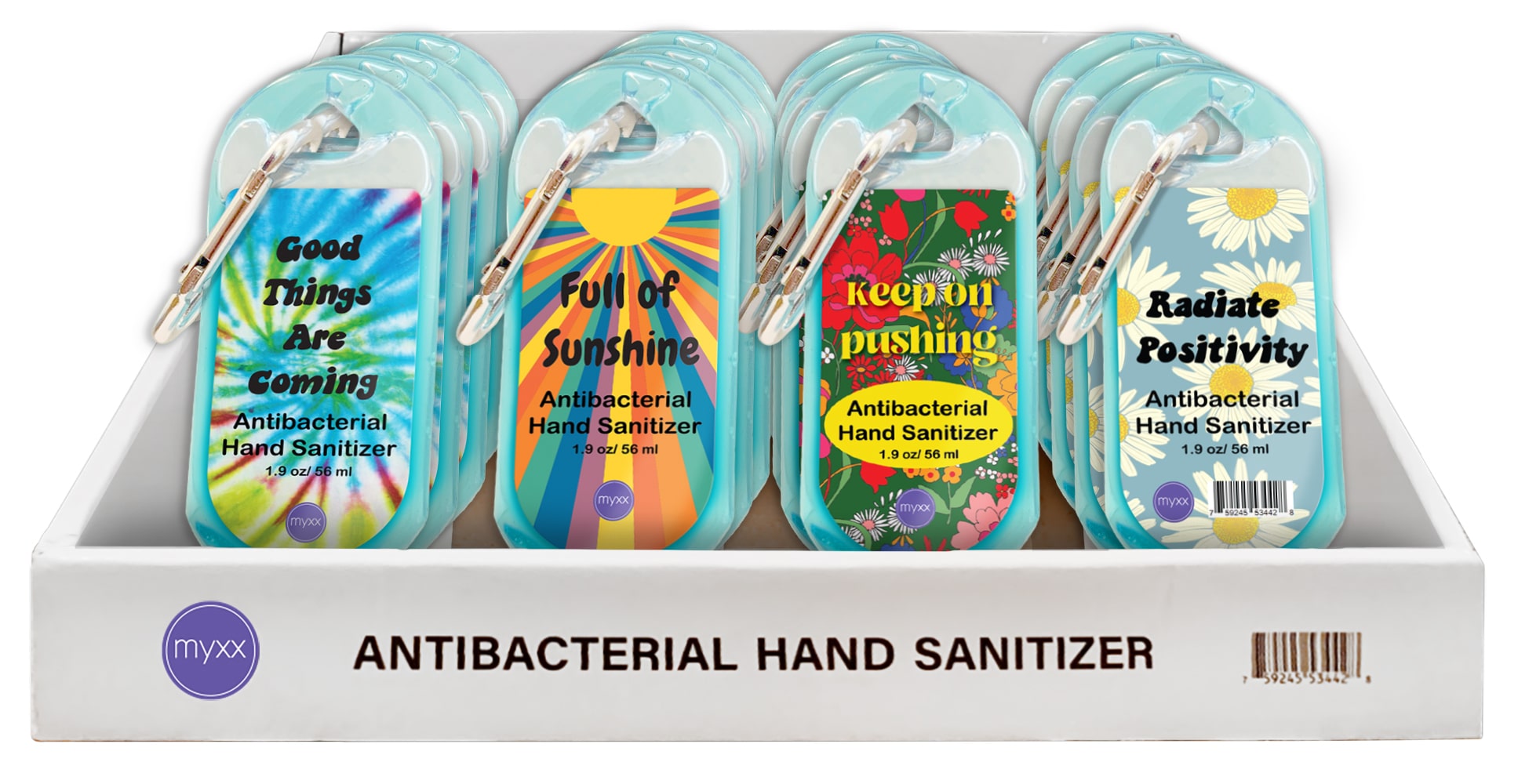
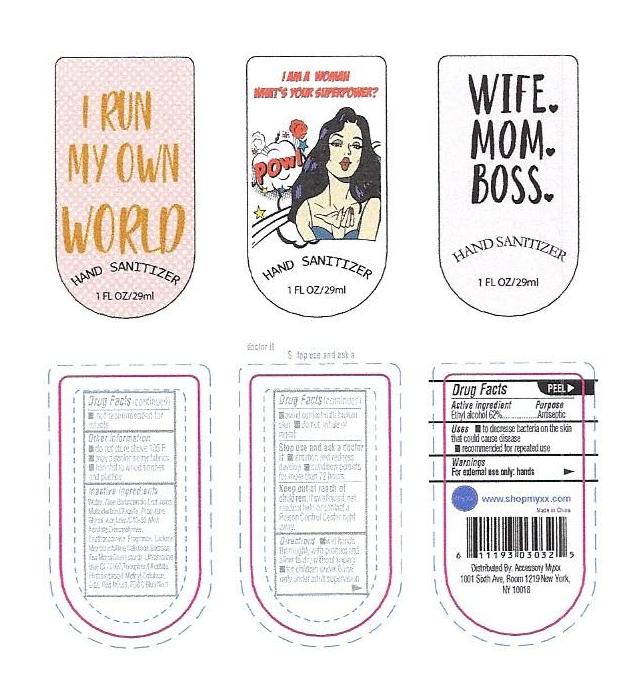
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
INSTANT HAND SANITIZER
instant hand sanitizer gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72619-2020 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 75 mL in 100 mL Inactive Ingredients Ingredient Name Strength AMINOMETHYL PROPANEDIOL (UNII: CZ7BU4QZJZ) 0.18 mL in 100 mL WATER (UNII: 059QF0KO0R) 28.42 mL in 100 mL GLYCERIN (UNII: PDC6A3C0OX) 1 mL in 100 mL CARBOMER 980 (UNII: 4Q93RCW27E) 0.35 mL in 100 mL FD&C BLUE NO. 1 (UNII: H3R47K3TBD) 0.0001 mL in 100 mL FRAGRANCE CLEAN ORC0600327 (UNII: 329LCV5BTF) 0.04 mL in 100 mL ALOE VERA LEAF (UNII: ZY81Z83H0X) 0.35 mL in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72619-2020-1 56 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 05/18/2020 2 NDC:72619-2020-2 500 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 05/18/2020 3 NDC:72619-2020-4 3 mL in 1 PACKET; Type 0: Not a Combination Product 05/18/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 05/18/2020 INSTANT HAND SANITIZER
instant hand sanitizer gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72619-1219 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 62 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) SUCROSE (UNII: C151H8M554) ZEA MAYS POLLEN (UNII: 74PD8J616H) ULTRAMARINE BLUE (UNII: I39WR998BI) 3-HEXYLOXYPROPYLENE GLYCOL (UNII: 3485P35DA4) (C10-C30)ALKYL METHACRYLATE ESTER (UNII: XH2FQZ38D8) GLYCERIN (UNII: PDC6A3C0OX) MALTODEXTRIN (UNII: 7CVR7L4A2D) D&C RED NO. 33 (UNII: 9DBA0SBB0L) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72619-1219-3 2.9 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 10/10/2018 2 NDC:72619-1219-2 56 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 10/10/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 10/10/2018 INSTANT HAND SANITIZER
instant hand sanitizer gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72619-1001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 62 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CARBOMER 940 (UNII: 4Q93RCW27E) AMINOMETHYL PROPANEDIOL (UNII: CZ7BU4QZJZ) GLYCERIN (UNII: PDC6A3C0OX) ALOE VERA LEAF (UNII: ZY81Z83H0X) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72619-1001-3 2.9 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 10/10/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 10/10/2018 Labeler - Acessory Myxx LLC (080553023) Establishment Name Address ID/FEI Business Operations Leeds (China) Biotechnology Co., Ltd 416037073 manufacture(72619-2020) Establishment Name Address ID/FEI Business Operations Nantong Health & Beyond Hygienic Products Inc. 421280161 manufacture(72619-1219) Establishment Name Address ID/FEI Business Operations NINGBO OCEANSTAR CHEMICAL PRODUCTS CO LTD 544493972 manufacture(72619-1001)