Label: MOBISYN PAIN RELIEVING- methyl salicylate and menthol gel
-
Contains inactivated NDC Code(s)
NDC Code(s): 73799-001-03 - Packager: Chrono Health Care dba Natures Health Care
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated May 16, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
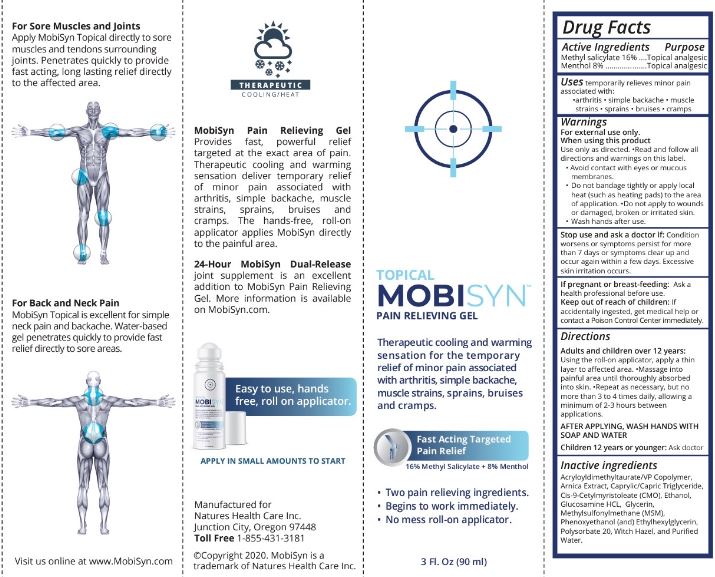
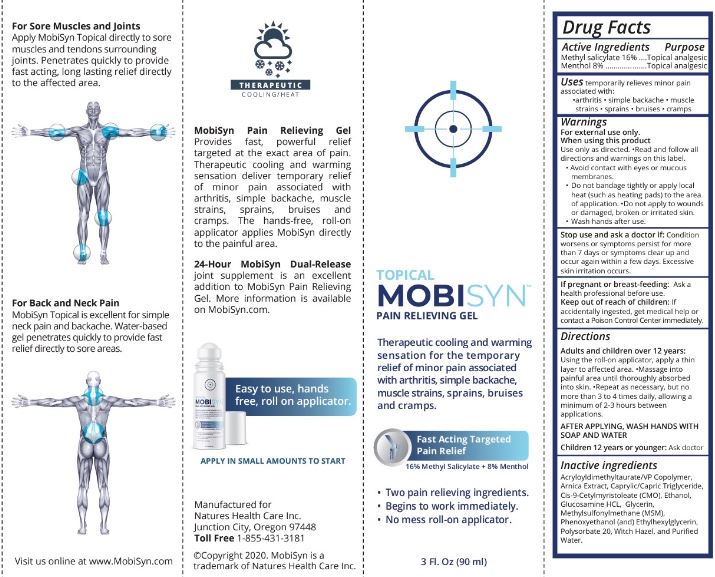
- Drug Facts
- Active Ingredients
- Purpose
- Uses
- Warnings
-
When using this product
Use only as directed. •Read and follow all directions and warnings on this label.
- Avoid contact with eyes or mucous membranes.
- Do not bandage tightly or apply local heat (such as heating pads) to the area of application. •Do not apply to wounds or damaged, broken or irritated skin.
- Wash hands after use.
- Stop use and ask a doctor if:
- If pregnant or breast-feeding:
- Keep out of reach of children:
-
Directions
Adults and children over 12 years:
Using the roll-on applicator, apply a thin layer to affected area. •Massage into painful area until thoroughly absorbed into skin. •Repeat as necessay, but no more than 3 to 4 times daily, allowing a minimum of 2-3 hours between applications.
AFTER APPLYING, WASH HANDS WITH SOAP AND WATER
Children 12 years or younger: Ask doctor
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MOBISYN PAIN RELIEVING
methyl salicylate and menthol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73799-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 16 mg in 100 mL MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) (MENTHOL, UNSPECIFIED FORM - UNII:L7T10EIP3A) MENTHOL, UNSPECIFIED FORM 8 mg in 100 mL Inactive Ingredients Ingredient Name Strength AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER (UNII: W59H9296ZG) GLUCOSAMINE HYDROCHLORIDE (UNII: 750W5330FY) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) GLYCERIN (UNII: PDC6A3C0OX) PHENOXYETHANOL (UNII: HIE492ZZ3T) ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) DIMETHYL SULFONE (UNII: 9H4PO4Z4FT) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) POLYSORBATE 20 (UNII: 7T1F30V5YH) WITCH HAZEL (UNII: 101I4J0U34) CETYL MYRISTOLEATE (UNII: 87P8K33Q5X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73799-001-03 1 in 1 CARTON 09/22/2020 1 90 mL in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 09/22/2020 Labeler - Chrono Health Care dba Natures Health Care (014996415) Establishment Name Address ID/FEI Business Operations Specialty Formulations and Manufacturing LLC 003989912 manufacture(73799-001)