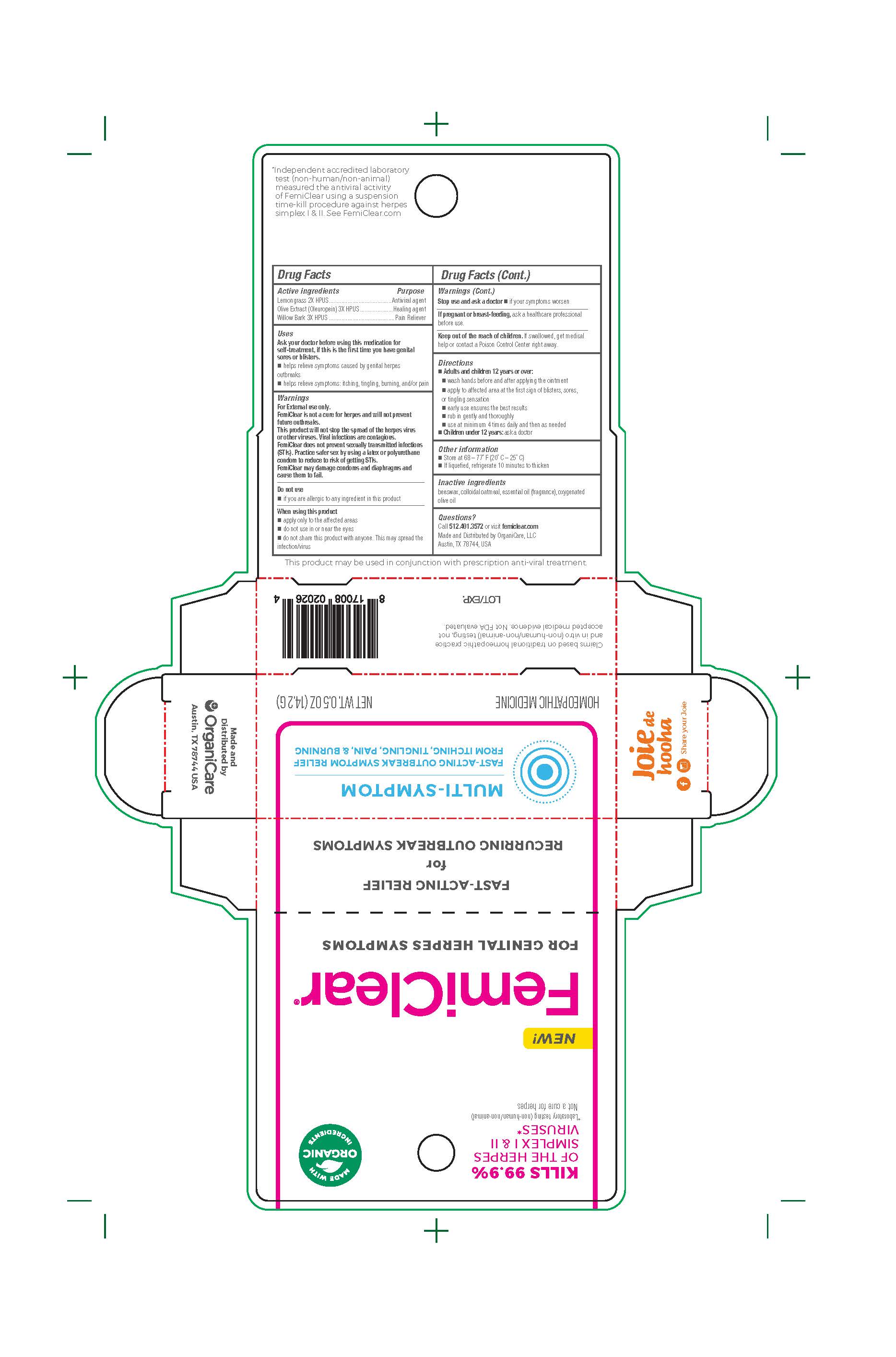
Label: FEMICLEAR FOR GENITAL HERPES SYMPTOMS- lemongrass with olive extract ointment
- NDC Code(s): 71042-020-14
- Packager: Organicare Nature's Science, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated January 11, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredients
- Purpose
- Uses
-
Warnings
For external use only.
FemiClear is not a cure for herpes and will not prevent future outbreaks.
This product will not stop the spread of herpes viruses or other viruses. Viral infections are contagious.
FemiClear does not prevent sexually transmitted infections (STIs). Practice safer sex by using latex or polyurethane condom to reduce risk of getting STIs.
FemiClear may damage condoms and diaphragms and cause ethem to fail
- Do not use
- When using this product
- Stop use and ask a doctor
- If pregnant or breast-feeding
- Keep out of the reach of children
-
Directions
Adults and children 12 years of age and older:
- wash hands before and after applying the ointment
- apply to affected area t teh first sign of blister, sores, or tingling sensation
- early use ensures the best results
- rub in gently and thoroughly
- use at minimum 4 times daily and then as needed
Children under 12 years: ask a doctor
- Inactve ingredients
- Questions? Call 512.401.3572 or visit femiclear.com
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
FEMICLEAR FOR GENITAL HERPES SYMPTOMS
lemongrass with olive extract ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71042-020 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WILLOW BARK (UNII: S883J9JDYX) (WILLOW BARK - UNII:S883J9JDYX) WILLOW BARK 3 [hp_X] in 100 g OLEA EUROPAEA FRUIT VOLATILE OIL (UNII: 8E7358CX1J) (OLEUROPEIN - UNII:2O4553545L) OLEUROPEIN 3 [hp_X] in 100 g EAST INDIAN LEMONGRASS OIL (UNII: UP0M8M3VZW) (EAST INDIAN LEMONGRASS OIL - UNII:UP0M8M3VZW) EAST INDIAN LEMONGRASS OIL 2 [hp_X] in 100 g Inactive Ingredients Ingredient Name Strength OATMEAL (UNII: 8PI54V663Y) YELLOW WAX (UNII: 2ZA36H0S2V) OLIVE OIL (UNII: 6UYK2W1W1E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71042-020-14 1 in 1 CARTON 07/01/2020 1 14.2 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 07/01/2020 Labeler - Organicare Nature's Science, LLC (044204745) Registrant - Organicare Nature's Science, LLC (044204745) Establishment Name Address ID/FEI Business Operations Organicare Nature's Science, LLC 044204745 manufacture(71042-020)