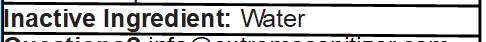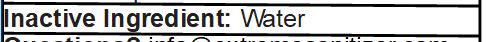Label: EXTREME SANITIZER- hand sanitizer liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 77868-0001-1, 77868-0001-2, 77868-0001-3, 77868-0001-4, view more77868-0001-5 - Packager: Earth Wind Fire L.L.C. / Extreme Sanitizer®
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 28, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Extreme Sanitizer®

Extreme Sanitizer®
Kills 99.99% of Germs in 15 Seconds
Effective Hand Sanitizer
Advanced Formula
KILLS CORONAVIRUS IN 15 SECONDS
Hypoallergenic and ALCOHOL-FREE
Drug Facts
Active Ingredient:Alkylbenzyldimethylammonium
Chloride (Quaternary Ammonium) 0.13%…Antiseptic
Use: For hand sanitation. No water wash.
WARNING: Avoid eye contact, flush with
water. Keep out of reach of children.
STOP use if redness or irritation occurs.
Call poison control if swallowed.
Directions:Apply spray to full cover hands
and rub. Repetition increases the effect.
Inactive Ingredient: Water
Questions? info@extremesanitizer.com
Other information: Store at 56 – 86 oFEarth Wind Fire L.L.C. The Woodlands, TX. 77382
www.extremesanitizer.com
2 FL OZ
(60 mL)Active Ingredient
Active Ingredient:Alkylbenzyldimethylammonium
Chloride (Quaternary Ammonium) 0.13%…Antiseptic
Dosage and administration
Active Ingredient:Alkylbenzyldimethylammonium
Chloride (Quaternary Ammonium) 0.13%…AntisepticUse: For hand sanitation. No water wash.
Directions:Apply spray to full cover hands
and rub. Repetition increases the effect.
Do Not Use Stop Use
WARNING: Avoid eye contact, flush with
water. Keep out of reach of children.
STOP use if redness or irritation occurs.
Call poison control if swallowed.
Stop Use
WARNING: Avoid eye contact, flush with
water. Keep out of reach of children.
STOP use if redness or irritation occurs.
Call poison control if swallowed.
-
INGREDIENTS AND APPEARANCE
EXTREME SANITIZER
hand sanitizer liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:77868-0001 Route of Administration CUTANEOUS, TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 1.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:77868-0001-1 60 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 03/30/2020 2 NDC:77868-0001-2 250 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 03/30/2020 3 NDC:77868-0001-3 500 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 03/30/2020 4 NDC:77868-0001-4 1000 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 03/30/2020 5 NDC:77868-0001-5 3875.41 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 03/30/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 03/30/2020 Labeler - Earth Wind Fire L.L.C. / Extreme Sanitizer® (111867055)