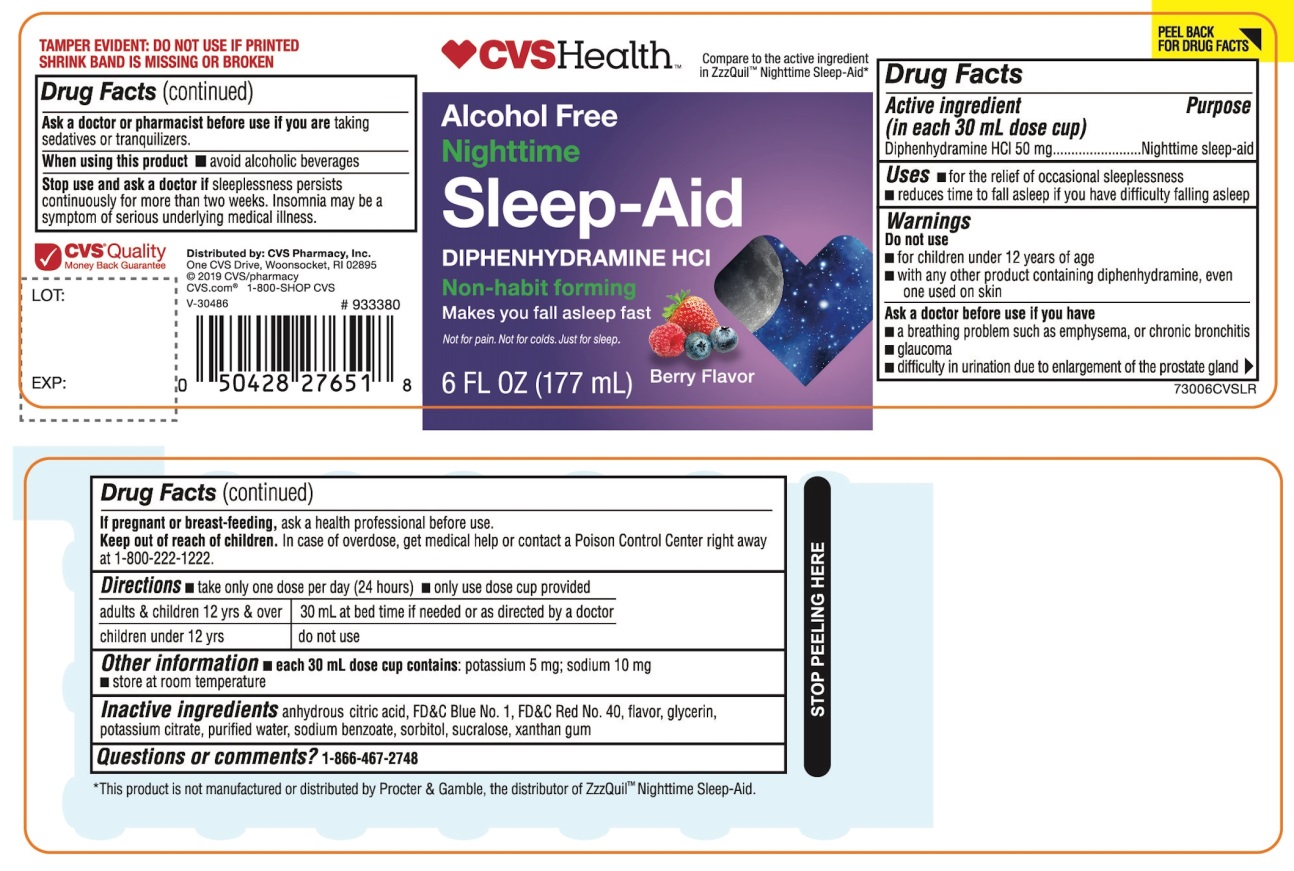
Label: CVS HEALTH NIGHTTIME SLEEP-AID- diphenhydramine hydrochloride liquid
- NDC Code(s): 59779-893-06
- Packager: CVS Pharmacy,Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 14, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient (in each 30 mL)
- Purpose
- Uses
-
Warnings
Do not use
- ▪
- for children under 12 years of age
- ▪
- with any other product containing diphenhydramine, even one used on skin
- ▪
- with other drugs that cause drowsiness such as antihistamines and nighttime cold/flu products
Ask a doctor before use if you have
- ▪
- a breathing problem such as asthma, emphysema, or chronic bronchitis
- ▪
- glaucoma
- ▪
- difficulty in urination due to enlargement of the prostate gland
- ▪
- heart disease
Ask a doctor or pharmacist before use if you are taking sedatives or tranquilizers or any other sleep- aid.
When using this product
- ▪
- avoid alcoholic beverages and other drugs that cause drowsiness
- ▪
- drowsiness will occur
- ▪
- be careful when driving a motor vehicle or operating machinery
- Directions
- Other information
- Inactive ingredients
-
PRINCIPAL DISPLAY PANEL
CVS Health™
NDC 59779-893-06
Compare to the active ingredient in ZzzQuil® Nighttime Sleep-Aid*
Alcohol Free
NighttimeSleep-Aid
DIPHENHYDRAMINE HCl
Non-habit forming
- Not for treating cold or flu
Berry Flavor
Naturally and Artificially Flavored
6 FL OZ (177 mL)
FAILURE TO FOLLOW THESE WARNINGS COULD RESULT IN SERIOUS CONSEQUENCES.
TAMPER EVIDENT: DO NOT USE IF PRINTED SHRINK BAND IS MISSING OR BROKEN
*This product is not manufactured or distributed by Procter & Gamble, the distributor of ZzzQuil®
Distributed by: CVS Pharmacy, Inc.
One CVS Drive, Woonsocket, RI 02895
© 2019CVS/pharmacy
CVS.com® 1-800-SHOP CVS
V-30486
CVS® Quality
Money Back Guarantee
-
INGREDIENTS AND APPEARANCE
CVS HEALTH NIGHTTIME SLEEP-AID
diphenhydramine hydrochloride liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59779-893 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 50 mg in 30 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) GLYCERIN (UNII: PDC6A3C0OX) POTASSIUM CITRATE (UNII: EE90ONI6FF) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SORBITOL (UNII: 506T60A25R) SUCRALOSE (UNII: 96K6UQ3ZD4) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color PURPLE Score Shape Size Flavor BERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59779-893-06 177 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 09/22/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 09/22/2015 Labeler - CVS Pharmacy,Inc. (062312574)