Label: SE-NATAL 19 CHEWABLE- vitamins and minerals tablet, chewable
- NDC Code(s): 13925-117-01
- Packager: Seton Pharmaceuticals
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated June 7, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
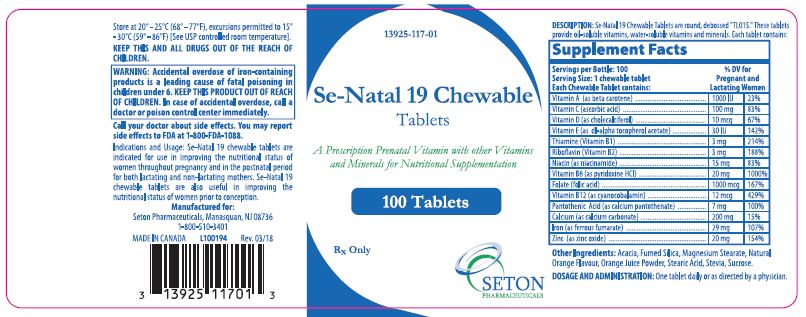
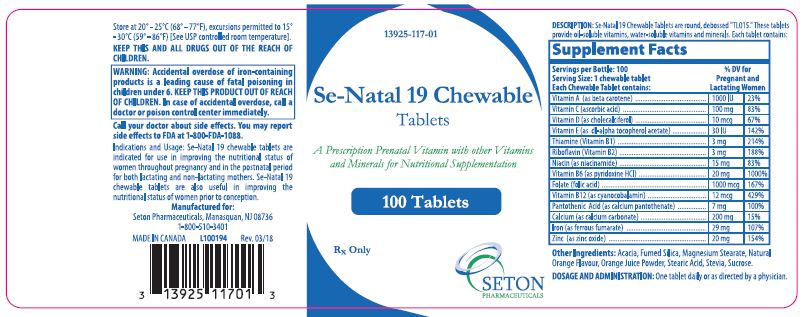
Se·Natal 19 Chewable tablets are round, debossed "TL015." These tablets provide oil-soluble vitamins, water-soluble vitamins and minerals. Each tablet contains:
Supplement Facts Servings per Bottle: 100 %DV For Pregnant and Lactating Women Serving Size 1 chewable tablet Each Tablet contains: Vitamin A (as beta carotene)
1000 IU 23% Vitamin C (calcium ascorbate) 100 mg 83% Vitamin D (as cholecalciferol) 10 mcg 67% Vitamin E (as dl-alpha tocopherol acetate) 30 IU 142% Thiamine (Vitamin B1) 3 mg 214% Riboflavin (Vitamin B2) 3 mg 188% Niacin (as niacinamide) 15 mg 83% Vitamin B6 (as pyridoxine HCl) 20 mg 1000% Folate (folic acid) 1000 mcg 167% Vitamin B12 (as cyanocobalamin) 12 mcg 429% Pantothenic Aicd (as calcium pantothenate) 7 mg 100% Calcium (as calcium carbonate) 200 mg 15% Iron (as ferrous fumarate) 29 mg 107% Zinc (as zinc oxide) 20 mg 154% -
CLINICAL PHARMACOLOGY
Oral iron is absorbed most efficiently when administered between meals. Iron is critical for normal hemogloblin syntheis to maintain oxygen transport, energy production and proper function of cells. Adequate amounts of iron are necessary for effective erythropoiesis. Iron also serves as a cofactor of several essential enzymes, including cytochromes, which are involved in electron transport. Folic acid is required for nucleoprotein synthesis and the maintenance of normal erythropoiesis. Folic acid is the precursor of tetrahydrofolic acid, which is involved as a cofactor for transformylation reactions in the biosynthesis of purines and thymidylates of nucleic acids. Deficiency of folic acid may account for the defective deoxyribonucleic acid (DNA) synthesis that leads to megaloblast formation and megablastic macrocytic anemias. Vitamin B12 is essential to growth cell reproduction, hematopoiesis, nucleic acid, and myelin synthesis. Deficiency may result in megaloblastic anemia or pernicious anemia.
- OTHER INGREDIENTS
-
INDICATIONS AND USAGE
Se-Natal 19 Chewable Tablets are indicated for use in improving the nutritional status of women throughout pregnancy and in the postnatal period for both lactating and non-lactating mothers. Se-Natal 19 Chewable Tablets are also useful in improving the nutritional status of women prior to conception.
- CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- ADVERSE REACTIONS
- DRUG INTERACTIONS
-
OVERDOSAGE
Symptoms:
Abdominal pain, metabolic acidosis, anuria, CNS damage, coma, convulsions, death, dehydration, diffuse vascular congestion, hepatic cirrhosis, hypotension, hypothermia, lethargy, nausea, vomiting, diarrhea, tarry stools, melena, hematemesis, tachycardia, hyperglycemia, drowsiness, pallor, cyanosis, lassitude, seizures, and shock.
- DOSAGE AND ADMINISTRATION
- NOTICE
- HOW SUPPLIED
- STORAGE
-
PHARMACIST
Dispense in a tight, light-resistant container as defined in the USP/INF with child-resistant closure.
Call your doctor about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Reserved for Professional
Recommendation.
KEEP THIS AND ALL DRUGS OUT OF THE REACH OF CHILDREN.
Rx Only
Manufactured For:
Seton Pharmaceuticals
Manasquan, NJ 08736
1-800-510-3401
MADE IN CANADACode 117-01
Rev. 03/18
SETON PHARMACEUTICALS
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SE-NATAL 19 CHEWABLE
vitamins and minerals tablet, chewableProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:13925-117 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FOLIC ACID (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) FOLIC ACID 1 mg .BETA.-CAROTENE (UNII: 01YAE03M7J) (.BETA.-CAROTENE - UNII:01YAE03M7J) .BETA.-CAROTENE 1000 [iU] CALCIUM ASCORBATE (UNII: 183E4W213W) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 100 mg CHOLECALCIFEROL (UNII: 1C6V77QF41) (CHOLECALCIFEROL - UNII:1C6V77QF41) CHOLECALCIFEROL 400 [iU] .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) (.ALPHA.-TOCOPHEROL, DL- - UNII:7QWA1RIO01) .ALPHA.-TOCOPHEROL, DL- 30 [iU] THIAMINE MONONITRATE (UNII: 8K0I04919X) (THIAMINE ION - UNII:4ABT0J945J) THIAMINE 3 mg RIBOFLAVIN (UNII: TLM2976OFR) (RIBOFLAVIN - UNII:TLM2976OFR) RIBOFLAVIN 3 mg NIACINAMIDE (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) NIACINAMIDE 15 mg PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE 20 mg CYANOCOBALAMIN (UNII: P6YC3EG204) (CYANOCOBALAMIN - UNII:P6YC3EG204) CYANOCOBALAMIN 12 ug CALCIUM PANTOTHENATE (UNII: 568ET80C3D) (PANTOTHENIC ACID - UNII:19F5HK2737) PANTOTHENIC ACID 7 mg CALCIUM CARBONATE (UNII: H0G9379FGK) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CATION 200 mg FERROUS FUMARATE (UNII: R5L488RY0Q) (FERROUS CATION - UNII:GW89581OWR) FERROUS CATION 29 mg ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 20 mg Inactive Ingredients Ingredient Name Strength ACACIA (UNII: 5C5403N26O) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) ORANGE JUICE (UNII: 5A9KE2L9L3) STEARIC ACID (UNII: 4ELV7Z65AP) STEVIA REBAUDIUNA LEAF (UNII: 6TC6NN0876) SUCROSE (UNII: C151H8M554) Product Characteristics Color BROWN Score no score Shape ROUND Size 12mm Flavor ORANGE Imprint Code TL015 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13925-117-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 07/01/2009 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 07/01/2009 Labeler - Seton Pharmaceuticals (828898002)