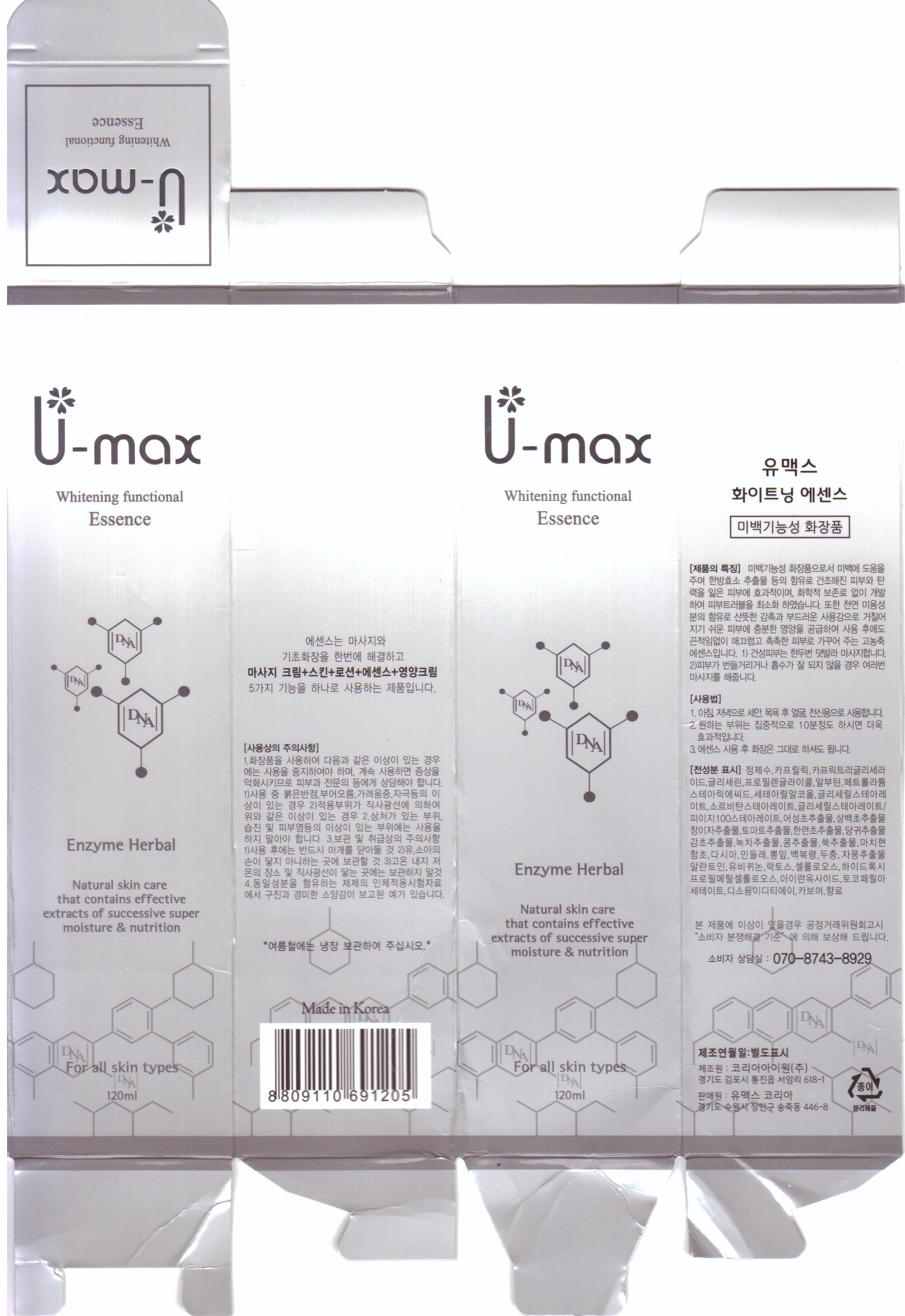
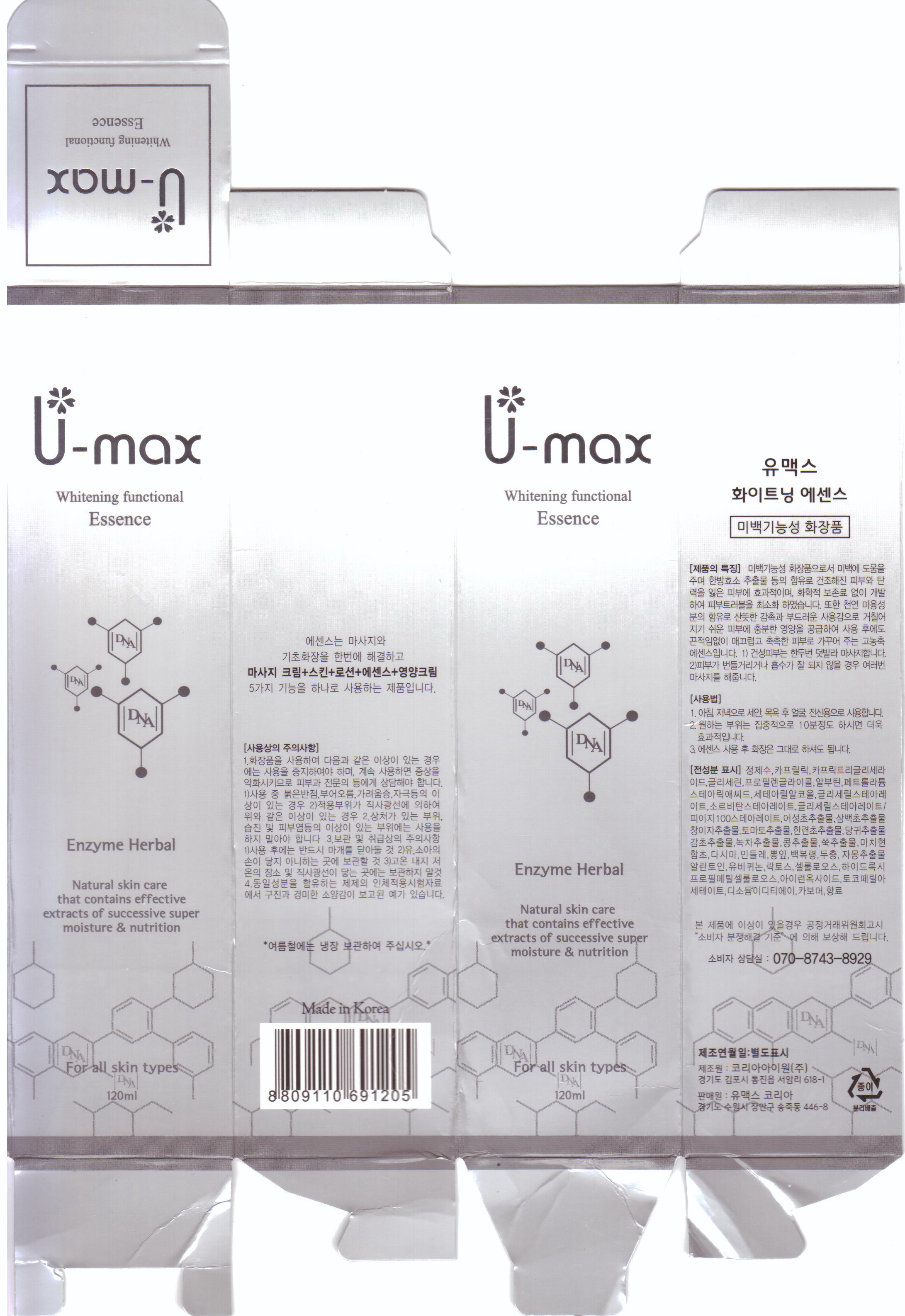
Label: U-MAX WHITENING FUNCTIONAL ESSENCE- allantoin cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 50795-3001-1 - Packager: VS Shinbi Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated June 4, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
-
INACTIVE INGREDIENT
inactive ingredient: water, glycerin, propylene glycol, arbutin, disodium EDTA, tocopheryl acetate, carbomer, caprylic triglyceride, petrolatum, stearic acid, cetearyl alcohol, glyceryl stearate, sorbitan stearate, houttuynia cordata extract, saururus chinensis extract, xanthium strumarium fruit extract, solanum lycopersicum fruit extract, eclipta prostrata leaf extract, angelica gigas root extract, glycyrrhiza glabra root extract, camellia sinensis leaf extract, hydrolyzed soybean extract, artemisia vulgaris extract, perfume, portulaca oleracea extract, salicornia herbacea extract, laminaria japonica extract, taraxacum officinale leaf extract, morus bombycis leaf extract, poria cocos extract, eucommia ulmoides leaf extract, citrus paradisi seed oil, ubiquinone, biosaccharide gum-1, hydroxypropyl methylcellulose, iron titanium oxide, cellulose
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- WARNINGS
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
U-MAX WHITENING FUNCTIONAL ESSENCE
allantoin creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50795-3001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALLANTOIN (UNII: 344S277G0Z) (ALLANTOIN - UNII:344S277G0Z) ALLANTOIN 0.1 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) HOUTTUYNIA CORDATA FLOWERING TOP (UNII: RH041UUZ22) XANTHIUM STRUMARIUM FRUIT (UNII: TN770YC17C) SOLANUM LYCOPERSICUM (UNII: 0243Q4990L) ANGELICA GIGAS ROOT (UNII: 32766B2FHX) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) ARTEMISIA VULGARIS ROOT (UNII: 32MP823R8S) ARBUTIN (UNII: C5INA23HXF) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MINERAL OIL (UNII: T5L8T28FGP) STEARIC ACID (UNII: 4ELV7Z65AP) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) LAMINARIA JAPONICA (UNII: WE98HW412B) TARAXACUM OFFICINALE (UNII: 39981FM375) CITRUS PARADISI SEED (UNII: 12F08874Y7) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) SORBITAN SESQUIOLEATE (UNII: 0W8RRI5W5A) GREEN TEA LEAF (UNII: W2ZU1RY8B0) PORTULACA OLERACEA WHOLE (UNII: D5J3623SV2) SALICORNIA EUROPAEA (UNII: 6ADL50JAKW) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50795-3001-1 120 mL in 1 BOTTLE, WITH APPLICATOR Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 06/04/2012 Labeler - VS Shinbi Co., Ltd. (557817055) Registrant - VS Shinbi Co., Ltd. (557817055) Establishment Name Address ID/FEI Business Operations VS Shinbi Co., Ltd. 557817055 manufacture