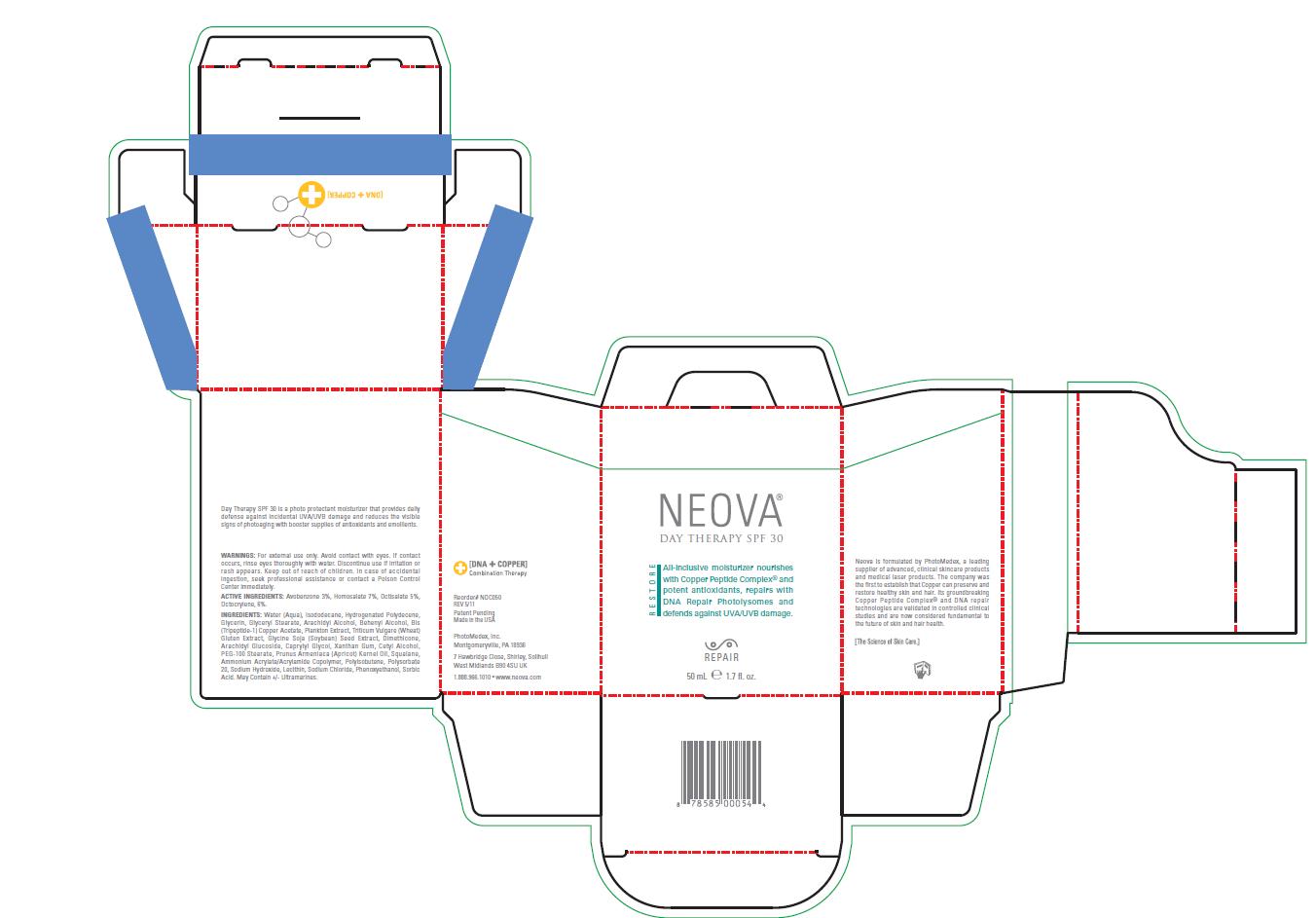
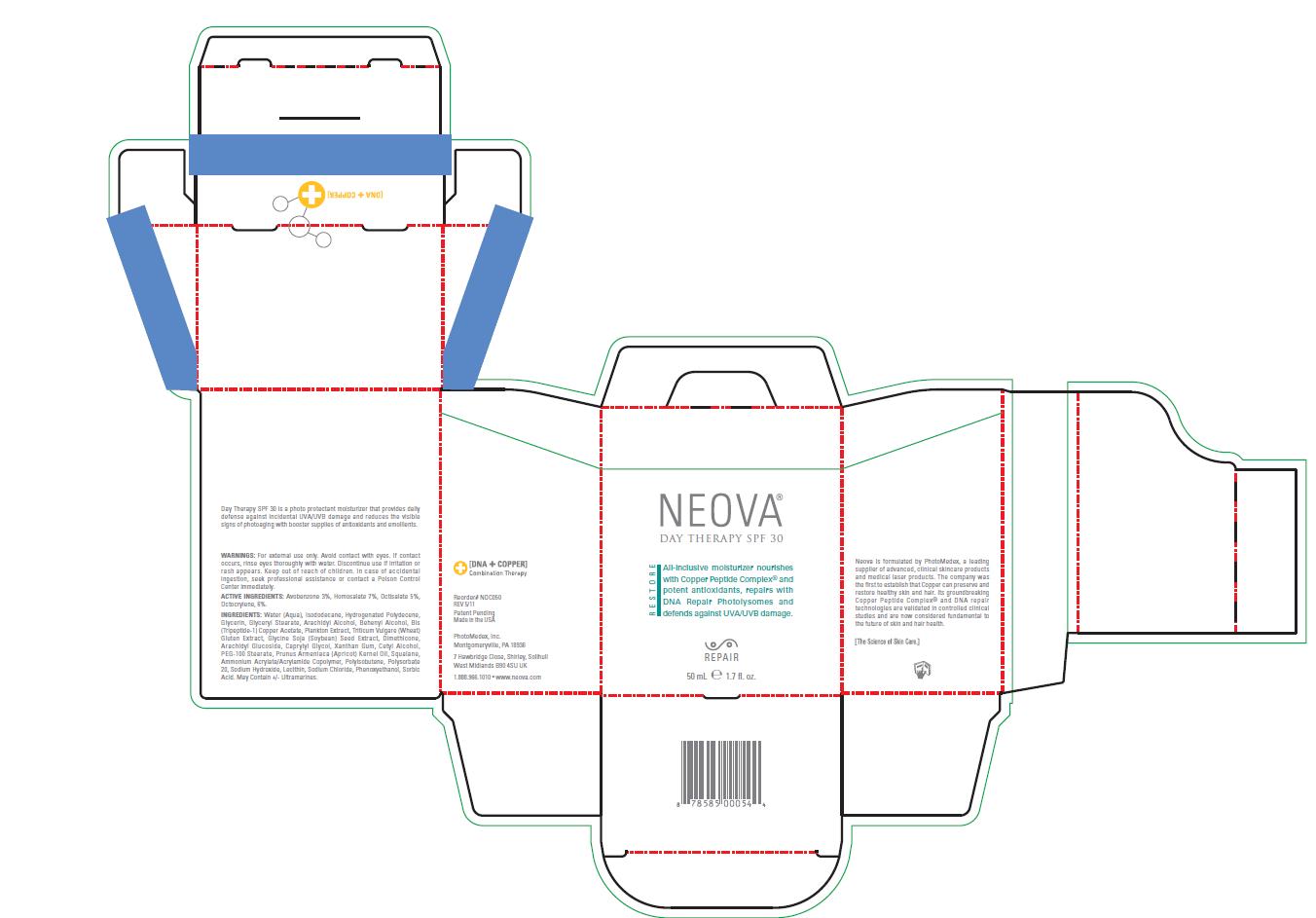
Label: NEOVA DAY THERAPY SPF 30- homosalate, octocrylene, octisalate, avobenzone lotion
-
Contains inactivated NDC Code(s)
NDC Code(s): 62362-184-05, 62362-184-50 - Packager: PhotoMedex, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 11, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Warnings
- Active Ingredients
-
Inactive Ingredients
Water (Aqua), Isododecane, Hydrogenated Polydecene,
Glycerin, Glyceryl Stearate, Arachidyl Alcohol, Behenyl Alcohol, Bis
(Tripeptide-1) Copper Acetate, Plankton Extract, Triticum Vulgare (Wheat)
Gluten Extract, Glycine Soja (Soybean) Seed Extract, Dimethicone,
Arachidyl Glucoside, Caprylyl Glycol, Xanthan Gum, Cetyl Alcohol,
PEG-100 Stearate, Prunus Armeniaca (Apricot) Kernel Oil, Squalane,
Ammonium Acrylate/Acrylamide Copolymer, Polyisobutene, Polysorbate
20, Sodium Hydroxide, Lecithin, Sodium Chloride, Phenoxyethanol, Sorbic
Acid. May Contain +/- Ultramarines. - Image of Package Insert, box, and label
-
INGREDIENTS AND APPEARANCE
NEOVA DAY THERAPY SPF 30
homosalate, octocrylene, octisalate, avobenzone lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62362-184 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Homosalate (UNII: V06SV4M95S) (Homosalate - UNII:V06SV4M95S) Homosalate 7 mL in 100 mL Octocrylene (UNII: 5A68WGF6WM) (Octocrylene - UNII:5A68WGF6WM) Octocrylene 6 mL in 100 mL Octisalate (UNII: 4X49Y0596W) (Octisalate - UNII:4X49Y0596W) Octisalate 5 mL in 100 mL Avobenzone (UNII: G63QQF2NOX) (Avobenzone - UNII:G63QQF2NOX) Avobenzone 3 mL in 100 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Isododecane (UNII: A8289P68Y2) Hydrogenated Polydecene (550 MW) (UNII: U333RI6EB7) Glycerin (UNII: PDC6A3C0OX) Glyceryl Monostearate (UNII: 230OU9XXE4) Arachidyl Alcohol (UNII: 1QR1QRA9BU) Docosanol (UNII: 9G1OE216XY) Prezatide Copper Acetate (UNII: A3LEI4P1NB) Soybean (UNII: L7HT8F1ZOD) Dimethicone (UNII: 92RU3N3Y1O) Arachidyl Glucoside (UNII: 6JVW35JOOJ) Caprylyl Glycol (UNII: 00YIU5438U) Xanthan Gum (UNII: TTV12P4NEE) Cetyl Alcohol (UNII: 936JST6JCN) PEG-100 Stearate (UNII: YD01N1999R) Apricot Kernel Oil (UNII: 54JB35T06A) Squalane (UNII: GW89575KF9) Polyisobutylene (1300 MW) (UNII: 241BN7J12Y) Polysorbate 20 (UNII: 7T1F30V5YH) Sodium Hydroxide (UNII: 55X04QC32I) Lecithin, Soybean (UNII: 1DI56QDM62) Sodium Chloride (UNII: 451W47IQ8X) Phenoxyethanol (UNII: HIE492ZZ3T) Sorbic Acid (UNII: X045WJ989B) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62362-184-05 1 in 1 BOX 1 NDC:62362-184-50 50 mL in 1 BOTTLE, PUMP Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 01/11/2012 Labeler - PhotoMedex, Inc. (054503875) Establishment Name Address ID/FEI Business Operations PhotoMedex, Inc. 054503875 manufacture




 PkgInsertComboTherapypg2.jpg
PkgInsertComboTherapypg2.jpg DayTherapy50mLImpt.jpg
DayTherapy50mLImpt.jpg DayTherapy50mLBox.jpg
DayTherapy50mLBox.jpg