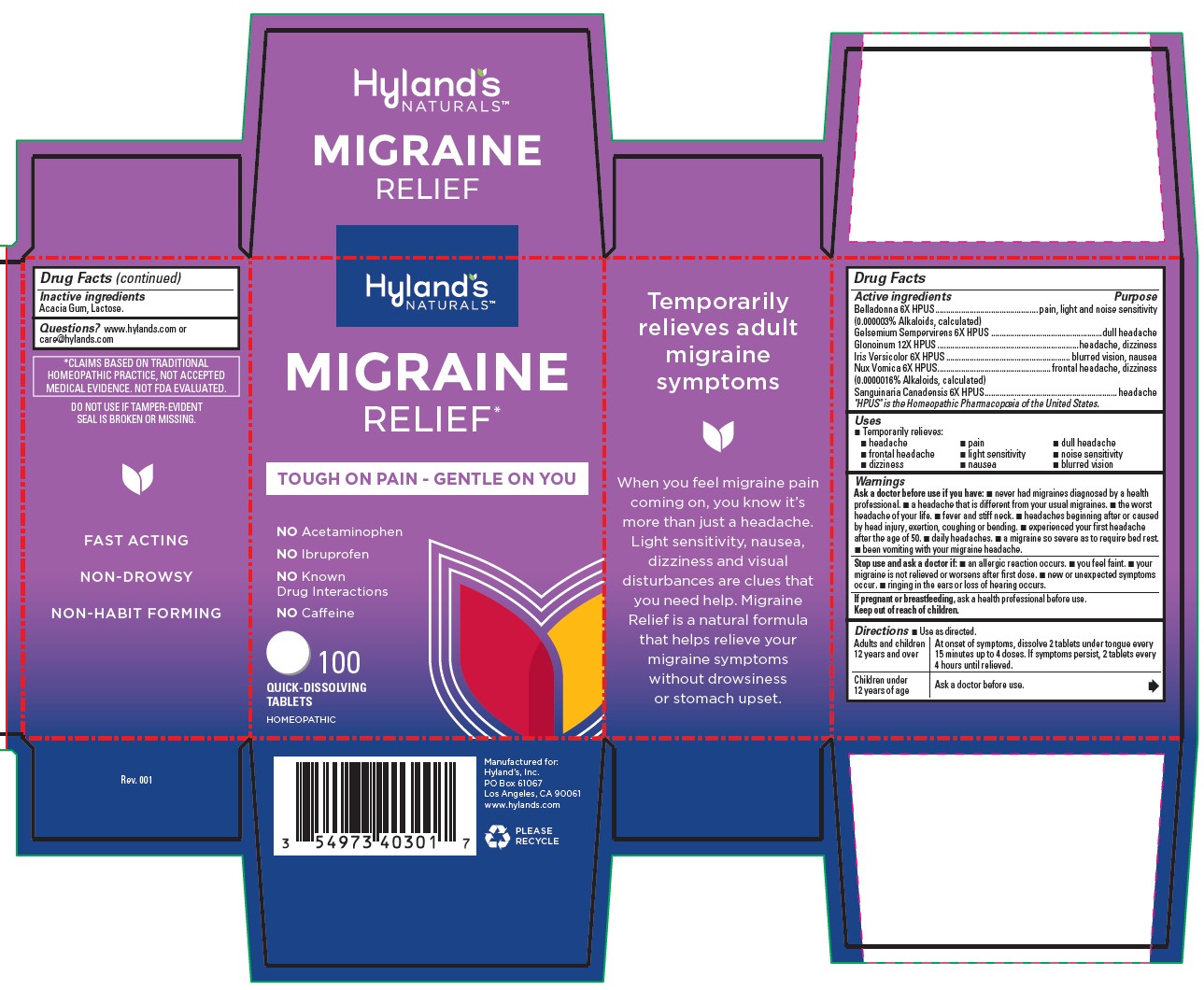
Label: MIGRAINE RELIEF- gelsemium sempervirens root, strychnos nux-vomica seed, iris versicolor root, sanguinaria canadensis root, atropa belladonna and nitroglycerin tablet
- NDC Code(s): 54973-4030-1
- Packager: Hyland's Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated December 13, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- PURPOSE
-
Drug Facts
Active ingredients
Active ingredients
Purpose
Belladonna 6X HPUS
(0.0000003% Alkaloids, calculated)
pounding pain, light and noise sensitivity
Gelsemium 6X HPUS
dull headache
Glonoinum 12X HPUS
throbbing headache, dizziness
Iris Versicolor 6X HPUS
headache, blurred vision, nausea
Nux Vomica 6X HPUS
(0.0000016% Alkaloids, calculated)
frontal headache, dizziness
Sanguinaria Canadensis 6X HPUS
headache
"HPUS" indicates that the active ingredients are in the official Homeopathic
Pharmacopoeia of the United States.
- Uses
-
Warnings
Ask a doctor before use if you have
■ never had migraines diagnosed by a health professional. ■ a headache that is different from your usual migraines. ■ the worst headache of your life. ■ fever and stiff neck. ■ headaches beginning after or caused by head injury, exertion, coughing or bending. ■ experienced your first headache after the age of 50. ■ daily headaches. a migraine so severe as to require bed rest. ■ been vomiting with your migraine headache.
- Directions
- Drug Facts (continued)
- *CLAIMS BASED ON TRADITIONAL HOMEOPATHIC PRACTICE, NOT ACCEPTED MEDICAL EVIDENCE. NOT FDA EVALUATED.
- DO NOT USE IF TAMPER-EVIDENT SEAL IS BROKEN OR MISSING.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MIGRAINE RELIEF
gelsemium sempervirens root, strychnos nux-vomica seed, iris versicolor root, sanguinaria canadensis root, atropa belladonna and nitroglycerin tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54973-4030 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NITROGLYCERIN (UNII: G59M7S0WS3) (NITROGLYCERIN - UNII:G59M7S0WS3) NITROGLYCERIN 12 [hp_X] GELSEMIUM SEMPERVIRENS ROOT (UNII: 639KR60Q1Q) (GELSEMIUM SEMPERVIRENS ROOT - UNII:639KR60Q1Q) GELSEMIUM SEMPERVIRENS ROOT 6 [hp_X] SANGUINARIA CANADENSIS ROOT (UNII: N9288CD508) (SANGUINARIA CANADENSIS ROOT - UNII:N9288CD508) SANGUINARIA CANADENSIS ROOT 6 [hp_X] IRIS VERSICOLOR ROOT (UNII: X43D4L3DQC) (IRIS VERSICOLOR ROOT - UNII:X43D4L3DQC) IRIS VERSICOLOR ROOT 6 [hp_X] STRYCHNOS NUX-VOMICA SEED (UNII: 269XH13919) (STRYCHNOS NUX-VOMICA SEED - UNII:269XH13919) STRYCHNOS NUX-VOMICA SEED 6 [hp_X] ATROPA BELLADONNA (UNII: WQZ3G9PF0H) (ATROPA BELLADONNA - UNII:WQZ3G9PF0H) ATROPA BELLADONNA 6 [hp_X] Inactive Ingredients Ingredient Name Strength ACACIA (UNII: 5C5403N26O) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) Product Characteristics Color white (White to Off White) Score no score Shape ROUND Size 9mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54973-4030-1 1 in 1 CARTON 03/30/2020 1 100 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 03/30/2020 Labeler - Hyland's Inc (008316655) Establishment Name Address ID/FEI Business Operations Hyland's Inc. 008316655 manufacture(54973-4030) , pack(54973-4030) , label(54973-4030)