Label: MESALAMINE enema
- NDC Code(s): 45802-098-28, 45802-098-46, 45802-098-51
- Packager: Padagis Israel Pharmaceuticals Ltd
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 1, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
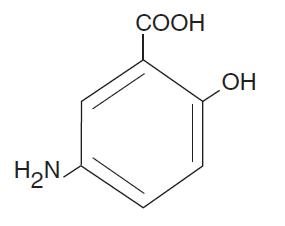
The active ingredient in Mesalamine Rectal Suspension, USP Enema, a disposable (60 mL) unit, is mesalamine, also known as 5-aminosalicylic acid (5-ASA). Chemically, mesalamine is 5-amino-2-hydroxybenzoic acid.
The empirical formula is C7H7NO3, representing a molecular weight of 153.14. The structural formula is:
Each rectal suspension enema unit contains 4 grams of mesalamine. In addition to mesalamine the preparation contains the inactive ingredients carbomer 934P, edetate disodium, potassium acetate, potassium metabisulfite, purified water and xanthan gum. Sodium benzoate is added as a preservative. The disposable unit consists of an applicator tip protected by a polyethylene cover and lubricated with USP white petrolatum. The unit has a one-way valve to prevent back flow of the dispensed product.
-
CLINICAL PHARMACOLOGY
Each mesalamine rectal suspension enema delivers up to 4 g of mesalamine to the left side of the colon.
The mechanism of action of mesalamine (and sulfasalazine) is not fully understood, but appears to be a topical anti-inflammatory effect on colonic epithelial cells. Mucosal production of arachidonic acid (AA) metabolites, both through the cyclooxygenase pathways, i.e., prostanoids, and through the lipoxygenase pathways, i.e., leukotrienes (LTs) and hydroxyeicosatetraenoic acids (HETEs) is increased in patients with ulcerative colitis, and it is possible that mesalamine diminishes inflammation by blocking cyclooxygenase and inhibiting prostaglandin (PG) production in the colon.
Preclinical Toxicology -
Preclinical studies have shown the kidney to be the major target organ for mesalamine toxicity. Adverse renal function changes were observed in rats after a single 600 mg/kg oral dose, but not after a 200 mg/kg dose. Gross kidney lesions, including papillary necrosis, were observed after a single oral >900 mg/kg dose, and after I.V. doses of > 214 mg/kg. Mice responded similarly. In a 13-week oral (gavage) dose study in rats, the high dose of 640 mg/kg/day mesalamine caused deaths, probably due to renal failure, and dose-related renal lesions (papillary necrosis and/or multifocal tubular injury) were seen in most rats given the high dose (males and females) as well as in males receiving lower doses 160 mg/kg/day. Renal lesions were not observed in the 160 mg/kg/day female rats. Minimal tubular epithelial damage was seen in the 40 mg/kg/day males and was reversible. In a six-month oral study in dogs, the no-observable dose level of mesalamine was 40 mg/kg/day and doses of 80 mg/kg/day and higher caused renal pathology similar to that described for the rat. In a combined 52-week toxicity and 127-week carcinogenicity study in rats, degeneration in kidneys was observed at doses of 100 mg/kg/day and above admixed with diet for 52 weeks, and at 127 weeks increased incidence of kidney degeneration and hyalinization of basement membranes and Bowman's capsule were seen at 100 mg/kg/day and above. In the 12-month eye toxicity study in dogs, Keratoconjunctivitis Sicca (KCS) occurred at oral doses of 40 mg/kg/day and above. The oral preclinical studies were done with a highly bioavailable suspension where absorption throughout the gastrointestinal tract occurred. The human dose of 4 grams represents approximately 80 mg/kg but when mesalamine is given rectally as a suspension, absorption is poor and limited to the distal colon (see Pharmacokinetics). Overt renal toxicity has not been observed (see ADVERSE REACTIONS and PRECAUTIONS), but the potential must be considered.
Pharmacokinetics -
Mesalamine administered rectally as mesalamine rectal suspension enema is poorly absorbed from the colon and is excreted principally in the feces during subsequent bowel movements. The extent of absorption is dependent upon the retention time of the drug product, and there is considerable individual variation. At steady state, approximately 10 to 30% of the daily 4-gram dose can be recovered in cumulative 24-hour urine collections. Other than the kidney, the organ distribution and other bioavailability characteristics of absorbed mesalamine in man are not known. It is known that the compound undergoes acetylation but whether this process takes place at colonic or systemic sites has not been elucidated.
Whatever the metabolic site, most of the absorbed mesalamine is excreted in the urine as the N-acetyl-5-ASA metabolite. The poor colonic absorption of rectally administered mesalamine is substantiated by the low serum concentration of 5-ASA and N-acetyl-5-ASA seen in ulcerative colitis patients after dosage with mesalamine. Under clinical conditions patients demonstrated plasma levels 10 to 12 hours post mesalamine administration of 2 µg/mL, about two-thirds of which was the N-acetyl metabolite. While the elimination half-life of mesalamine is short (0.5 to 1.5 h), the acetylated metabolite exhibits a half-life of 5 to 10 hours [U. Klotz, Clin. Pharmacokin. 10:285-302 (1985)]. In addition, steady state plasma levels demonstrated a lack of accumulation of either free or metabolized drug during repeated daily administrations.
Efficacy -
In a placebo-controlled, international, multicenter trial of 153 patients with active distal ulcerative colitis, proctosigmoiditis or proctitis, mesalamine rectal suspension enema reduced the overall disease activity index (DAI) and individual components as follows:
EFFECT OF TREATMENT ON SEVERITY OF DISEASE DATA FROM U.S.- CANADA TRIAL COMBINED RESULTS OF EIGHT CENTERS
Activity Indices, mean
N
Baseline
Day 22
End Point
Change Baseline
to End Point*
Overall DAI
Mesalamine Rectal Suspension Enema
76
7.42
4.05†
3.37‡
-55.07%‡
Placebo
77
7.40
6.03
5.83
-21.58%
Stool Frequency
Mesalamine Rectal Suspension Enema
1.58
1.11§
1.01†
-0.57§
Placebo
1.92
1.47
1.50
-0.41
Rectal Bleeding
Mesalamine Rectal Suspension Enema
1.82
0.59‡
0.51‡
-1.30‡
Placebo
1.73
1.21
1.11
-0.61
Mucosal Inflammation
Mesalamine Rectal Suspension Enema
2.17
1.22†
0.96‡
-1.21†
Placebo
2.18
1.74
1.61
-0.56
Physician’s Assessment
of Disease Severity
Mesalamine Rectal Suspension Enema
1.86
1.13‡
0.88‡
-0.97‡
Placebo
1.87
1.62
1.55
-0.30
Each parameter has a 4-point scale with a numerical rating:
0 = normal, 1 = mild, 2 = moderate, 3 = severe. The four parameters are added together to produce a maximum overall DAI of 12.
* Percent change for overall DAI only (calculated by taking the average of the change for each individual patient).
† Significant mesalamine rectal suspension enema /placebo difference. p<0.01
‡ Significant mesalamine rectal suspension enema /placebo difference. p<0.001
§ Significant mesalamine rectal suspension enema /placebo difference. p<0.05
Differences between mesalamine rectal suspension enema and placebo were also statistically different in subgroups of patients on concurrent sulfasalazine and in those having an upper disease boundary between 5 and 20 or 20 and 40 cm. Significant differences between mesalamine rectal suspension enema and placebo were not achieved in those subgroups of patients on concurrent prednisone or with an upper disease boundary between 40 and 50 cm.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
Hypersensitivity Reactions
Sulfite-Related Reactions
Mesalamine rectal suspension enema contains potassium metabisulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown but probably low. Sulfite sensitivity is seen more frequently in asthmatic or in atopic nonasthmatic persons.
Epinephrine is the preferred treatment for serious allergic or emergency situations even though epinephrine injection contains sodium or potassium metabisulfite with the above-mentioned potential liabilities. The alternatives to using epinephrine in a life-threatening situation may not be satisfactory. The presence of a sulfite(s) in epinephrine injection should not deter the administration of the drug for treatment of serious allergic or other emergency situations.
Sulfasalazine-Associated Reactions
Hypersensitivity reactions have been reported in patients taking sulfasalazine. Some patients may have a similar reaction to mesalamine rectal suspension enema or to other compounds that contain or are converted to mesalamine.
As with sulfasalazine, mesalamine-induced hypersensitivity reactions may present as internal organ involvement, including myocarditis, pericarditis, nephritis, hepatitis, pneumonitis and hematologic abnormalities. Evaluate patients immediately if signs or symptoms of a hypersensitivity reaction are present. Discontinue mesalamine rectal suspension enema if an alternative etiology for the signs and symptoms cannot be established.
Renal Impairment
Renal impairment, including minimal change disease, acute and chronic interstitial nephritis, and renal failure have been reported in patients given products that contain mesalamine or are converted to mesalamine. In animal studies, the kidney was the principal organ of mesalamine toxicity.
Evaluate the risks and benefits of using mesalamine rectal suspension enema in patients with known renal impairment or a history of renal disease or taking concomitant nephrotoxic drugs. Mesalamine is known to be substantially excreted by the kidney, and the risk of adverse reactions may be greater in patients with impaired renal function. Evaluate renal function in all patients prior to initiation and periodically while on mesalamine rectal suspension enema therapy. Discontinue mesalamine rectal suspension enema if renal function deteriorates while on therapy.
Mesalamine-Induced Acute Intolerance Syndrome
Mesalamine has been associated with an acute intolerance syndrome that may be difficult to distinguish from a flare of inflammatory bowel disease. Although the exact frequency of occurrence cannot be ascertained, it has occurred in 3% of patients in controlled clinical trials of mesalamine or sulfasalazine. Symptoms include cramping, acute abdominal pain and bloody diarrhea, sometimes fever, headache, and rash. Monitor patients for worsening of these symptoms while on treatment. If acute intolerance syndrome is suspected, promptly discontinue treatment with mesalamine rectal suspension enema.
-
PRECAUTIONS
Hepatic Failure
There have been reports of hepatic failure in patients with pre-existing liver disease who have been administered other products containing mesalamine. Evaluate the risks and benefits of using mesalamine rectal suspension enema in patients with known liver impairment.
Severe Cutaneous Adverse Reactions
Severe cutaneous adverse reactions, including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), drug reaction with eosinophilia and systemic symptoms (DRESS), and acute generalized exanthematous pustulosis (AGEP) have been reported with the use of mesalamine (see ADVERSE REACTIONS). Discontinue mesalamine rectal suspension enema at the first signs or symptoms of severe cutaneous adverse reactions or other signs of hypersensitivity and consider further evaluation.
Photosensitivity
Patients with pre-existing skin conditions such as atopic dermatitis and atopic eczema have reported more severe photosensitivity reactions. Advise patients to avoid sun exposure, wear protective clothing, and use a broad-spectrum sunscreen when outdoors.
Nephrolithiasis
Cases of nephrolithiasis have been reported with the use of mesalamine, including stones with 100% mesalamine content. Mesalamine-containing stones are radiotransparent and undetectable by standard radiography or computed tomography (CT). Ensure adequate hydration during treatment.
Information for Patients
Urine Discoloration
Advise patients that urine may become discolored reddish-brown while taking mesalamine rectal suspension enema when it comes in contact with surfaces or water treated with hypochlorite-containing bleach. If discolored urine is observed, advise patients to observe their urine flow. Report to the healthcare provider only if urine is discolored on leaving the body, before contact with any surface or water (e.g., in the toilet).
Interference with Laboratory Tests
Use of mesalamine may lead to spuriously elevated test results when measuring urinary normetanephrine by liquid chromatography with electrochemical detection because of the similarity in the chromatograms of normetanephrine and mesalamine’s main metabolite, N-acetyl-5-aminosalicylic acid (N-Ac-5-ASA). Consider an alternative, selective assay for normetanephrine.
Drug Interactions
Nephrotoxic Agents, Including Non-Steroidal Anti-Inflammatory Drugs
The concurrent use of mesalamine with known nephrotoxic agents, including non-steroidal anti-inflammatory drugs (NSAIDs), may increase the risk of nephrotoxicity. Monitor patients taking nephrotoxic drugs for changes in renal function and mesalamine-related adverse reactions.
Azathioprine or 6-Mercaptopurine
The concurrent use of mesalamine with azathioprine or 6-mercaptopurine and/or any other drugs known to cause myelotoxicity may increase the risk for blood disorders, bone marrow failure, and associated complications. If concomitant use of mesalamine rectal suspension enema and azathioprine or 6-mercaptopurine cannot be avoided, monitor blood tests, including complete blood cell counts and platelet counts.
Carcinogenesis, Mutagenesis, Impairment of Fertility -
Mesalamine caused no increase in the incidence of neoplastic lesions over controls in a 2-year study of Wistar rats fed up to 320 mg/kg/day of mesalamine admixed with diet. Mesalamine is not mutagenic to Salmonella typhimurium tester strains TA98, TA100, TA1535, TA1537, TA1538. There were no reverse mutations in an assay using E. coli strain WP2UVRA. There were no effects in an in vivo mouse micronucleus assay at 600 mg/kg and in an in vivo sister chromatid exchange at doses up to 610 mg/kg. No effects on fertility were observed in rats receiving up to 320 mg/kg/day. The oligospermia and infertility in men associated with sulfasalazine has very rarely been reported among patients treated with mesalamine.
Pregnancy -
Teratologic studies have been performed in rats and rabbits at oral doses up to five and eight times respectively, the maximum recommended human dose, and have revealed no evidence of harm to the embryo or the fetus. There are, however, no adequate and well-controlled studies in pregnant women for either sulfasalazine or 5-ASA. Because animal reproduction studies are not always predictive of human response, 5-ASA should be used during pregnancy only if clearly needed.
Nursing Mothers -
It is not known whether mesalamine or its metabolite(s) are excreted in human milk. As a general rule, nursing should not be undertaken while a patient is on a drug since many drugs are excreted in human milk.
Geriatric Use -
Clinical trials of mesalamine rectal suspension enema did not include sufficient numbers of patients aged 65 years and over to determine whether they respond differently from younger patients. Reports from uncontrolled clinical studies and postmarketing reporting systems suggested a higher incidence of blood dyscrasias (i.e., agranulocytosis, neutropenia and pancytopenia) in patients receiving mesalamine-containing products such as mesalamine rectal suspension enema who were 65 years or older compared to younger patients, which may also be associated with ulcerative colitis, use of interacting drugs, or reduced renal function.
Consider monitoring complete blood cell counts and platelet counts in elderly patients during treatment with mesalamine rectal suspension enema, especially if used concomitantly with anticoagulants. In general, consider the greater frequency of decreased hepatic, renal, or cardiac function, and of concurrent disease or other drug therapy in elderly patients when prescribing mesalamine rectal suspension enema.
- To report SUSPECTED ADVERSE REACTIONS, contact Padagis® at 1-866-634-9120 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
-
ADVERSE REACTIONS
Clinical Adverse Experience -
Mesalamine rectal suspension enema is usually well tolerated. Most adverse effects have been mild and transient.
ADVERSE REACTIONS OCCURRING IN MORE THAN 0.1% OF MESALAMINE RECTAL SUSPENSION ENEMA TREATED PATIENTS (COMPARISON TO PLACEBO)
MESALAMINE RECTAL SUSPENSION ENEMA
PLACEBO
SYMPTOM
N=815
N
%
N=128
N
%
Abdominal Pain/Cramps/Discomfort
66
8.10
10
7.81
Headache
53
6.50
16
12.50
Gas/Flatulence
50
6.13
5
3.91
Nausea
47
5.77
12
9.38
Flu
43
5.28
1
0.78
Tired/Weak/Malaise/Fatigue
28
3.44
8
6.25
Fever
26
3.19
0
0.00
Rash/Spots
23
2.82
4
3.12
Cold/Sore Throat
19
2.33
9
7.03
Diarrhea
17
2.09
5
3.91
Leg/Joint Pain
17
2.09
1
0.78
Dizziness
15
1.84
3
2.34
Bloating
12
1.47
2
1.56
Back Pain
11
1.35
1
0.78
Pain on Insertion of Enema Tip
11
1.35
1
0.78
Hemorrhoids
11
1.35
0
0.00
Itching
10
1.23
1
0.78
Rectal Pain
10
1.23
0
0.00
Constipation
8
0.98
4
3.12
Hair Loss
7
0.86
0
0.00
Peripheral Edema
5
0.61
11
8.59
UTI/Urinary Burning
5
0.61
4
3.12
Rectal Pain/Soreness/Burning
5
0.61
3
2.34
Asthenia
1
0.12
4
3.12
Insomnia
1
0.12
3
2.34
In addition, the following adverse events have been identified during post-approval use of products which contain (or are metabolized to) mesalamine in clinical practice: nephrotoxicity, pancreatitis, fibrosing alveolitis, elevated liver enzymes, nephrogenic diabetes insipidus, intracranial hypertension and nephrolithiasis. Cases of pancreatitis and fibrosing alveolitis have been reported as manifestations of inflammatory bowel disease as well.
Published case reports and/or spontaneous post marketing surveillance have described rare instances of aplastic anemia, agranulocytosis, thrombocytopenia, eosinophilia, pancytopenia, neutropenia, oligospermia, and infertility in men. Anemia, leukocytosis and thrombocytosis can be part of the clinical presentation of inflammatory bowel disease.
Postmarketing cases of severe cutaneous adverse reactions (SJS/TEN, DRESS, and AGEP) and pleurisy/pleuritis have been reported.
Hair Loss
Mild hair loss characterized by "more hair in the comb" but no withdrawal from clinical trials has been observed in 7 of 815 mesalamine patients but none of the placebo-treated patients. In the literature there are at least six additional patients with mild hair loss who received either mesalamine or sulfasalazine. Retreatment is not always associated with repeated hair loss.
Postmarketing Experience
The following adverse reactions have been identified during post-approval use of mesalamine. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Renal Disorders
- •
- Urine discoloration occurring ex-vivo caused by contact of mesalamine including inactive metabolite, with surfaces or water treated with hypochlorite-containing bleach (see PRECAUTIONS: Information for Patients).
-
OVERDOSAGE
Mesalamine absorption from the colon is limited; however, mesalamine rectal suspension enema is an aminosalicylate, and symptoms of salicylate toxicity include nausea, vomiting and abdominal pain, tachypnea, hyperpnea, tinnitus, and neurologic symptoms (headache, dizziness, confusion, seizures). Severe salicylate intoxication may lead to electrolyte and blood pH imbalance and potentially to other organ (e.g., renal and liver) involvement. There is no specific antidote for mesalamine overdose. Correct fluid and electrolyte imbalance by the administration of appropriate intravenous therapy and maintain adequate renal function.
-
DOSAGE AND ADMINISTRATION
The recommended adult dosage of mesalamine rectal suspension enema in 60 mL units is one rectal instillation (4 grams) once a day, preferably at bedtime, and retained for approximately eight hours. While the effect of mesalamine rectal suspension enema may be seen within 3 to 21 days, the usual course of therapy would be from 3 to 6 weeks depending on symptoms and sigmoidoscopic findings. Studies available to date have not assessed if mesalamine rectal suspension enema will modify relapse rates after the 6-week short-term treatment. Mesalamine rectal suspension enema is for rectal use only.
Drink an adequate amount of fluids during treatment.
Patients should be instructed to shake the bottle well to make sure the suspension is homogeneous. The patient should remove the protective sheath from the applicator tip. Holding the bottle at the neck will not cause any of the medication to be discharged. The position most often used is obtained by lying on the left side (to facilitate migration into the sigmoid colon); with the lower leg extended and the upper right leg flexed forward for balance. An alternative is the knee-chest position. The applicator tip should be gently inserted in the rectum pointing toward the umbilicus. A steady squeezing of the bottle will discharge most of the preparation. The preparation should be taken at bedtime with the objective of retaining it all night. Patient instructions are included with every seven units.
-
HOW SUPPLIED
Mesalamine Rectal Suspension, USP Enema for rectal administration is an off-white to tan colored suspension. Each disposable enema bottle contains 4.0 grams of mesalamine in 60 mL aqueous suspension. Enema bottles are supplied in boxed, foil-wrapped trays as follows:
Carton of 7 Bottles - NDC 45802-098-51
Carton of 28 Bottles - NDC 45802-098-28
Combo Kit with 7 Bottles and Wipes - NDC 45802-923-41
Combo Kit with 28 Bottles and Wipes - NDC 45802-929-49
Mesalamine rectal suspension enemas are for rectal use only.
KEEP OUT OF REACH OF CHILDREN
Patient instructions are included.
Storage
Store at 20°-25°C (68°-77°F). [See USP Controlled Room Temperature]. Once the foil-wrapped unit of seven bottles is opened, all enemas should be used promptly as directed by your physician.
Contents of enemas removed from the foil pouch may darken with time. Slight darkening will not affect potency, however, enemas with dark brown contents should be discarded.
NOTE: Mesalamine rectal suspension enema will cause staining of direct contact surfaces, including but not limited to fabrics, flooring, painted surfaces, marble, granite, vinyl, and enamel. Take care in choosing a suitable location for administration of this product.
For more information call Padagis® at 1-866-634-9120.
Rx Only
Manufactured by Padagis®
Yeruham, Israel
www.padagis.com
Rev 11-23
2N900 RC PH13
-
PATIENT INSTRUCTIONS
How to Use this Medication
Best results are achieved if the bowel is emptied immediately before the medication is given.
NOTE: Mesalamine rectal suspension enema will cause staining of direct contact surfaces, including but not limited to fabrics, flooring, painted surfaces, marble, granite, vinyl, and enamel. Take care in choosing a suitable location for administration of this product.
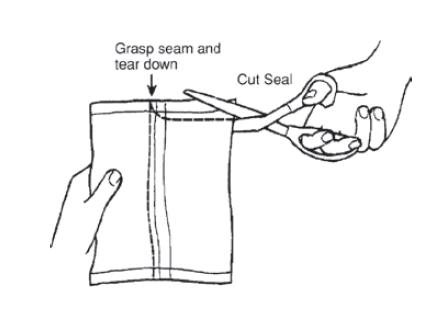
- 1.
- Remove the Bottles
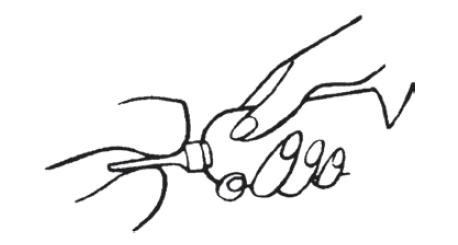
- a.
- Remove the bottles from the protective foil pouch by tearing or by using scissors as shown, being careful not to squeeze or puncture bottles. Mesalamine rectal suspension enema is an off-white to tan colored suspension. Once the foil-wrapped unit of seven bottles is opened, all enemas should be used promptly as directed by your physician.
- Contents of enemas removed from the foil pouch may darken with time. Slight darkening will not affect potency, however, enemas with dark brown contents should be discarded.
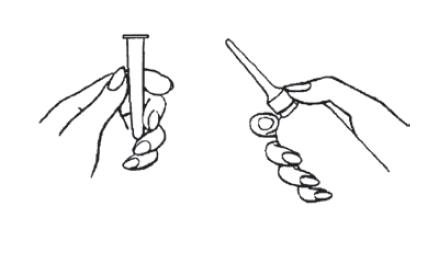
- 2.
- Prepare the Medication for Administration
- a.
- Shake the bottle well to make sure that the medication is thoroughly mixed.
- b.
- Remove the protective sheath from the applicator tip. Hold the bottle at the neck so as not to cause any of the medication to be discharged.
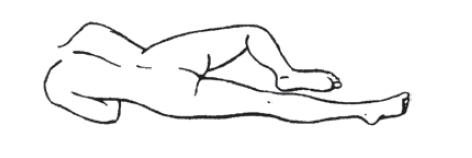
- 3.
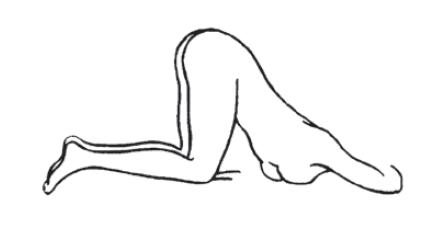
- Assume the Correct Body Position
- a.
- Best results are obtained by lying on the left side with the left leg extended and the right leg flexed forward for balance.
- b.
- An alternative to lying on the left side is the "knee-chest" position as shown here.
- 4.
- Administer the Medication
- a.
- Gently insert the lubricated applicator tip into the rectum to prevent damage to the rectal wall, pointed slightly toward the navel.
- b.
- Grasp the bottle firmly, then tilt slightly so that the nozzle is aimed toward the back, squeeze slowly to instill the medication.
- Steady hand pressure will discharge most of the medication. After administering, withdraw and discard the bottle.
- c.
- Remain in position for at least 30 minutes to allow thorough distribution of the medication internally. Retain the medication all night, if possible.
Rx Only
Manufactured by Padagis®
Yeruham, Israel
www.padagis.com
Rev 11-23
2N900 RC PH13
-
Principal Display Panel - Carton
Rx Only
NDC 45802-098-51
Mesalamine Rectal Suspension, USP Enema
4g/60 mL
Unit-Dose
For Rectal Use Only
7 Pack
7 x 60 mL UNIT-DOSE BOTTLES
The following image is a placeholder representing the product identifier that is either affixed or imprinted on the drug package label during the packaging operation.
-
INGREDIENTS AND APPEARANCE
MESALAMINE
mesalamine enemaProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:45802-098 Route of Administration RECTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MESALAMINE (UNII: 4Q81I59GXC) (MESALAMINE - UNII:4Q81I59GXC) MESALAMINE 4 g in 60 mL Inactive Ingredients Ingredient Name Strength CARBOMER HOMOPOLYMER TYPE B (ALLYL PENTAERYTHRITOL OR ALLYL SUCROSE CROSSLINKED) (UNII: K6MOM3T5YL) EDETATE DISODIUM (UNII: 7FLD91C86K) POTASSIUM ACETATE (UNII: M911911U02) POTASSIUM METABISULFITE (UNII: 65OE787Q7W) WATER (UNII: 059QF0KO0R) XANTHAN GUM (UNII: TTV12P4NEE) SODIUM BENZOATE (UNII: OJ245FE5EU) Product Characteristics Color WHITE (off white to tan) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:45802-098-51 7 in 1 CARTON 10/11/2007 1 60 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:45802-098-28 28 in 1 CARTON 02/12/2008 2 60 mL in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC:45802-098-46 60 mL in 1 BOTTLE; Type 0: Not a Combination Product 10/11/2007 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076751 10/11/2007 Labeler - Padagis Israel Pharmaceuticals Ltd (600093611)