Label: CYSTEX MAX UTI PAIN RELIEF- phenazopyridine hydrochloride tablet
- NDC Code(s): 69693-514-24
- Packager: Clarion Brands, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 22, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- INDICATIONS & USAGE
-
Warnings
- Do not exceed recommended dosage
- Long-term administration of phenazopyridine hydrochloride has induced neoplasia in rats (large intestine) and mice (liver). Although no association between phenazopyridine hydrochloride and human neoplasia has been reported, adequate epidemiological studies along these lines have not been conducted.
- Do not exceed recommended dosage
- Do not use
- Ask a doctor before use if you have
-
When using this product
- do not exceed recommended dosage
- stomach upset may occur, taking this product with or after meals may reduce stomach upset
- your urine will become reddish-orange in color. This is not harmful, but care should be taken to avoid staining clothing or other items.
- do not use for more than 2 days (12 tablets) without consulting a doctor
- do not exceed recommended dosage
- Stop use and ask a doctor if
-
PREGNANCY OR BREAST FEEDING
If pregnant or breast-feeding, ask a health professional before use. A pregnancy test and consultation with a healthcare professional if pregnancy is confirmed is recommended prior to use
Keep out of the reach of children. In case of overdose, get medical help or contact a Poison Control Center right away. - KEEP OUT OF REACH OF CHILDREN
- Directions
-
Other information
- WARNING: This product contains Phenazopyridine Hydrochloride, known to the state of California to cause cancer.
- this product can interfere with laboratory tests including urine, glucose (sugar), and ketones tests
- this product may stain soft contact lenses and other items if handled after touching tablets
- store at room temperature 59°F-86°F (15°C-30°C) in a dry place and protect from light
Tamper evident: Product is sealed within blisters. Do not use if any part of the blister is torn, open or damaged.
- WARNING: This product contains Phenazopyridine Hydrochloride, known to the state of California to cause cancer.
- Inactive ingredients
- Questions
-
PRINCIPAL DISPLAY PANEL
MAX STRENGTH
Cystex®
Urinary Health
Phenazopyridine Hydrochloride 99.5 mg
MAX UTI PAIN RELIEF
FAST-ACTING UTI PAIN RELIEF
Strongest UTI Pain Relief Available
for Fast-Acting Relief of Pain & Burning*
√ Relief of Pain, Burning, & Urgency
√ Targets Pain at the Source of the UTI
STARTS
WORKING
IN AS LITTLE AS
15 MINUTES
Cystex is not intended to replace a doctor's care.
24 Tablets
TAMPER EVIDENT: TABLETS SEALED IN BLISTER. DO NOT USE IF BLISTER IS OPENED OR DAMAGED.
1000284
REV XX/XX REESECODE
For more information on
Cystex Max Strength UTI Pain
Relief or other products, please
visit www.cystex.com or call
844-297-8394
Distributed by: Cystex LLC
811 Broad Street, Suite 600
Chattanooga, TN 37402 ©2024
*without a prescription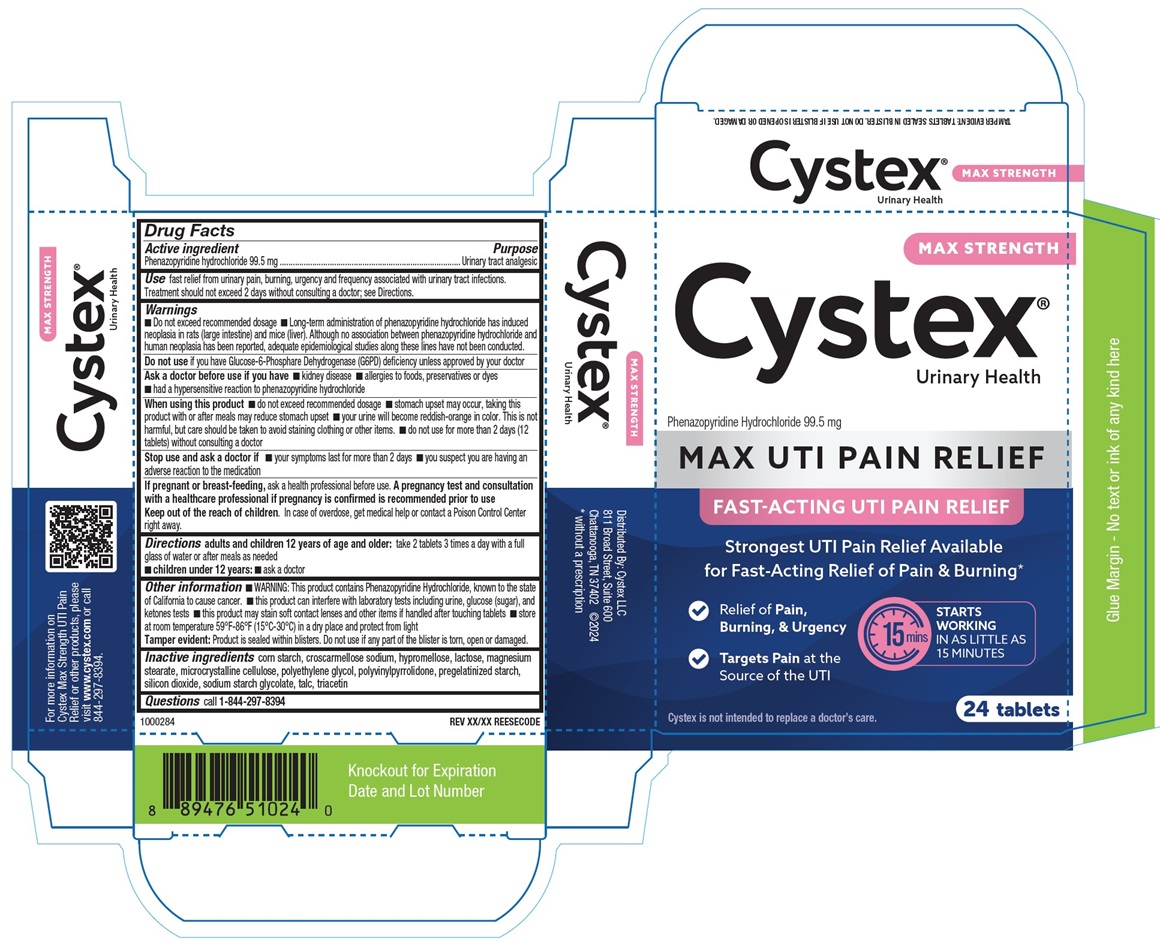
-
INGREDIENTS AND APPEARANCE
CYSTEX MAX UTI PAIN RELIEF
phenazopyridine hydrochloride tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69693-514 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Phenazopyridine Hydrochloride (UNII: 0EWG668W17) (Phenazopyridine - UNII:K2J09EMJ52) Phenazopyridine Hydrochloride 99.5 mg Inactive Ingredients Ingredient Name Strength Starch, Corn (UNII: O8232NY3SJ) Croscarmellose Sodium (UNII: M28OL1HH48) Hypromellose, Unspecified (UNII: 3NXW29V3WO) Lactose, Unspecified Form (UNII: J2B2A4N98G) Magnesium Stearate (UNII: 70097M6I30) Microcrystalline Cellulose (UNII: OP1R32D61U) Polyethylene Glycol, Unspecified (UNII: 3WJQ0SDW1A) Povidone, Unspecified (UNII: FZ989GH94E) Silicon Dioxide (UNII: ETJ7Z6XBU4) Sodium Starch Glycolate Type A (UNII: H8AV0SQX4D) Talc (UNII: 7SEV7J4R1U) Triacetin (UNII: XHX3C3X673) Product Characteristics Color RED Score no score Shape ROUND Size 10mm Flavor Imprint Code CYSTEX Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69693-514-24 1 in 1 CARTON 08/02/2024 1 24 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 08/02/2024 Labeler - Clarion Brands, LLC (079742703) Establishment Name Address ID/FEI Business Operations Pharbest Pharmaceuticals, Inc. 557054835 manufacture(69693-514)

