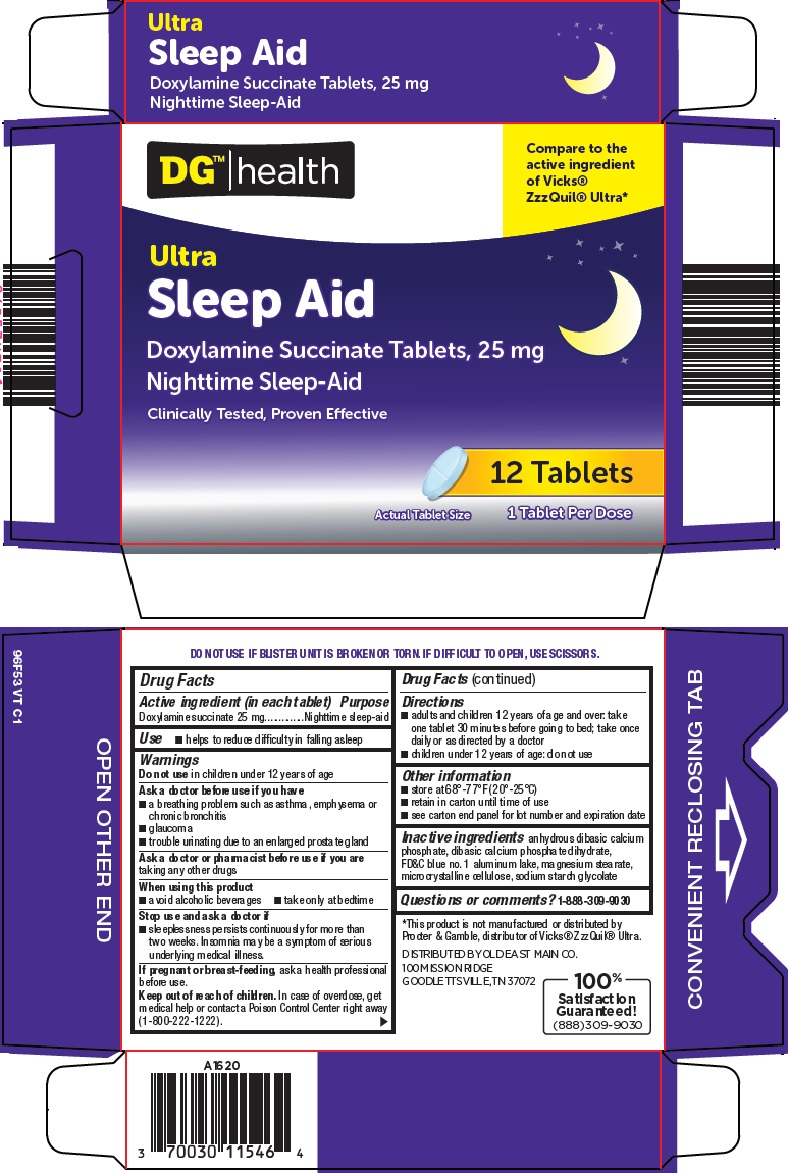
Label: DG HEALTH SLEEP AID- doxylamine succinate tablet
- NDC Code(s): 55910-909-53, 55910-909-62
- Packager: Dolgencorp Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 1, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each tablet)
- Purpose
- Use
-
Warnings
Ask a doctor before use if you have
- •
- a breathing problem such as asthma, emphysema or chronic bronchitis
- •
- glaucoma
- •
- trouble urinating due to an enlarged prostate gland
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
- Package/Label Principal Display Panel
-
INGREDIENTS AND APPEARANCE
DG HEALTH SLEEP AID
doxylamine succinate tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:55910-909 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOXYLAMINE SUCCINATE (UNII: V9BI9B5YI2) (DOXYLAMINE - UNII:95QB77JKPL) DOXYLAMINE SUCCINATE 25 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) Product Characteristics Color BLUE Score 2 pieces Shape OVAL Size 10mm Flavor Imprint Code L441 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55910-909-53 12 in 1 CARTON 05/23/2023 1 1 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:55910-909-62 24 in 1 CARTON 05/17/2023 2 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA040167 05/17/2023 Labeler - Dolgencorp Inc (068331990)