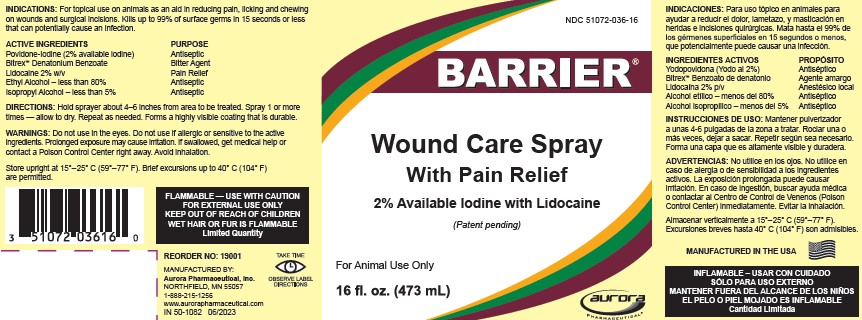
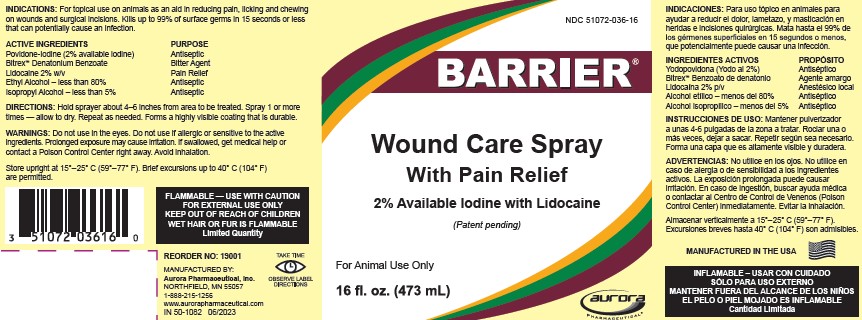
Label: BARRIER WOUND CARE WITH PAIN RELIEF- povidone-iodine, lidocaine, and alcohol solution
- NDC Code(s): 51072-036-01, 51072-036-16
- Packager: Aurora Pharmaceutical, Inc.
- Category: OTC ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated June 24, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- INDICATIONS
- DESCRIPTION
- DIRECTIONS
-
WARNINGS
Do not use in the eyes. Do not use if you are allergic or sensitive to the active ingredients. Prolonged exposure may cause irritation. If swallowed, get medical help or contact a Poison Control Center right away. Avoid inhalation.
Store upright at 15°–25° C (59°–77° F). Brief excursions up to 40° C (104° F) are permitted.
- BOXED WARNING (What is this?)
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 473 mL Bottle Label
-
INGREDIENTS AND APPEARANCE
BARRIER WOUND CARE WITH PAIN RELIEF
povidone-iodine, lidocaine, and alcohol solutionProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:51072-036 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Povidone-Iodine (UNII: 85H0HZU99M) (IODINE - UNII:9679TC07X4) IODINE 2 g in 100 mL Lidocaine (UNII: 98PI200987) (Lidocaine - UNII:98PI200987) Lidocaine 2 g in 100 mL ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 80 mL in 100 mL ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 5 mL in 100 mL Product Characteristics Color brown (brown) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51072-036-16 473 mL in 1 BOTTLE 2 NDC:51072-036-01 3790 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 10/15/2010 Labeler - Aurora Pharmaceutical, Inc. (832848639) Establishment Name Address ID/FEI Business Operations Aurora Pharmaceutical, Inc. 832848639 MANUFACTURE