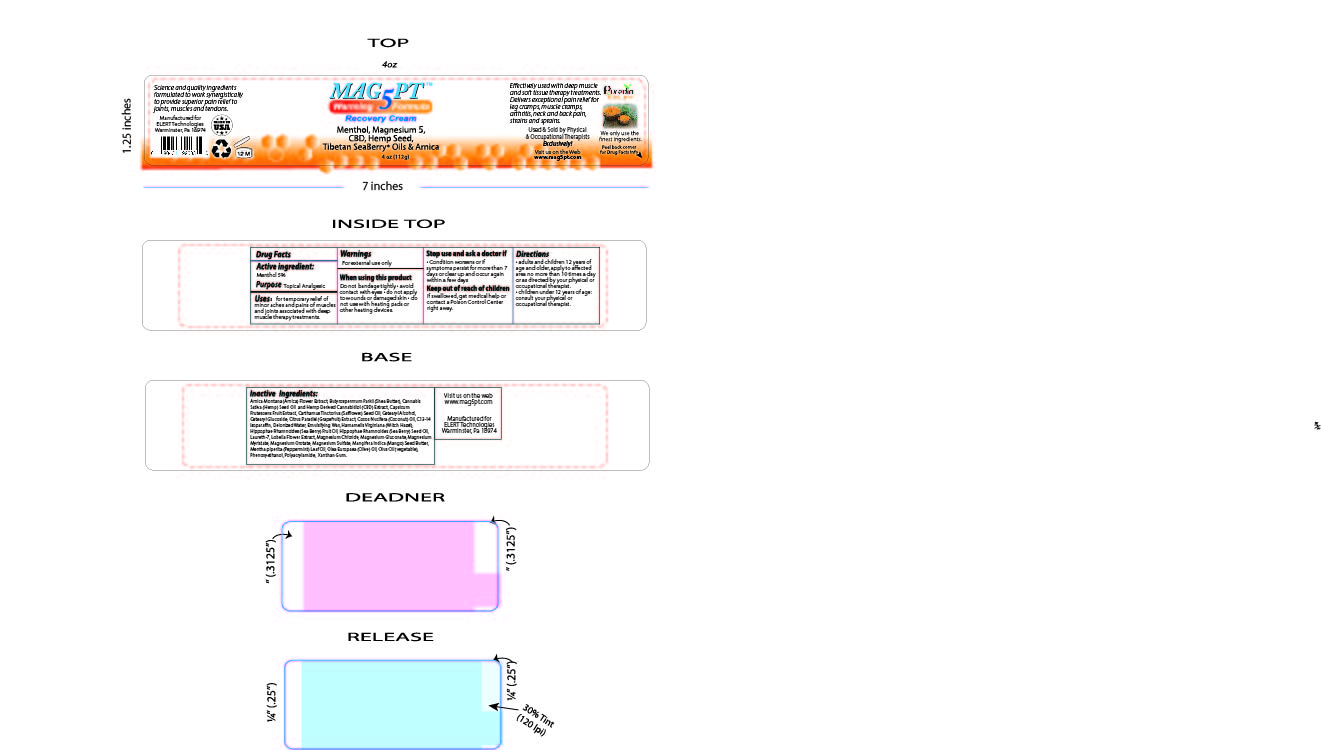
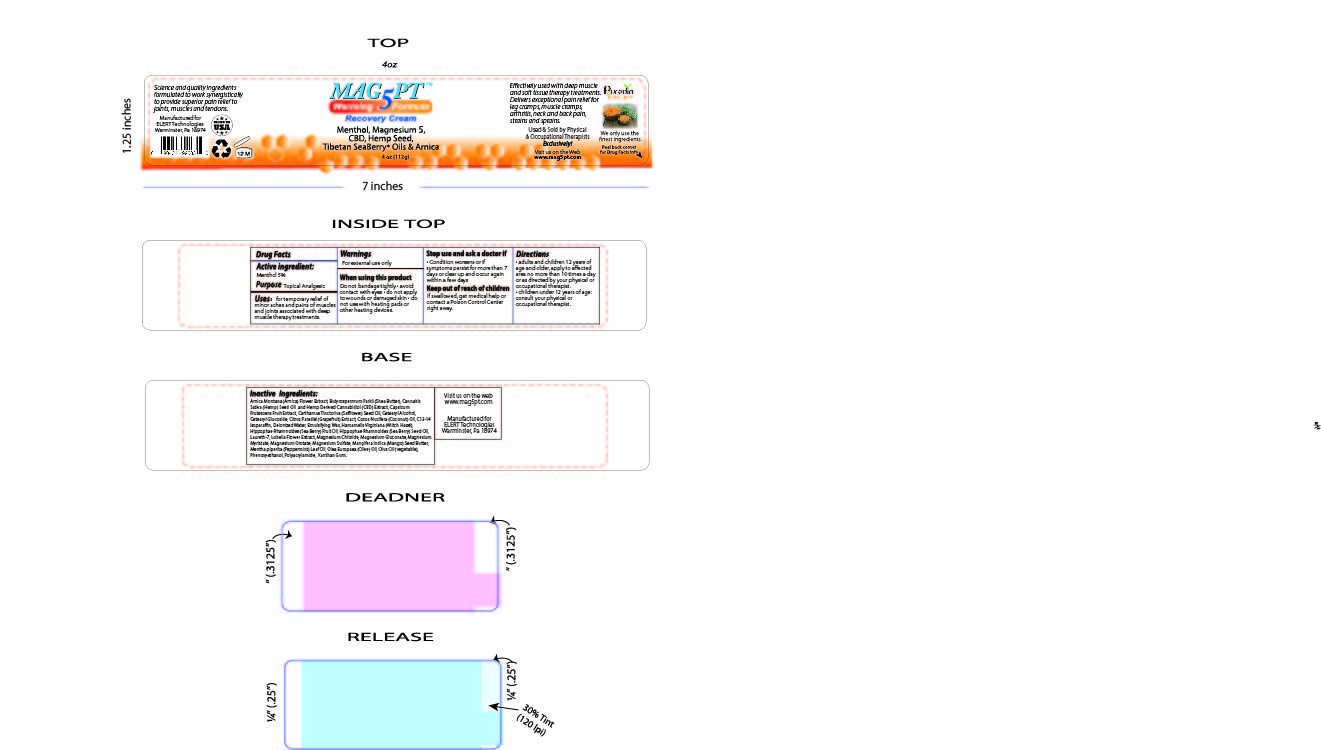
Label: MAG 5 PT WARMING FORMULA- menthol cream
- NDC Code(s): 76348-511-04, 76348-511-05
- Packager: RENU LABORATORIES, INC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated February 5, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- WHEN USING
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
-
INACTIVE INGREDIENT
Arnica Montana (Arnica) Flower Extract, Butyrospermum Parkii (Shea) Butter, Cannabis Sativa (Hemp) Seed Oil, Cetearyl Alcohol, Cetearyl Glucoside, Cocos Nucifera (Coconut) Oil, Deionized Water, Emulsifying Wax, Fragrance, Hamamelis Virginiana (Witch Hazel), Hemp Derived Cannabidiol (CBD) Extract, Laureth-7, Lobelia Inflata (Lobelia) Extract, Magnesium Chloride, Magnesium Citrate, Magnesium Gluconate, Magnesium Myristate, Magnesium Sulfate, Mangifera Indica (Mango) Seed Buter, Mentha Piperita (Peppermint) Oil, Olea Europaea (Olive) Oil, Olus Oil (Vegetable), Phenoxyethanol, Polyacrylamide C13-14 Isoparaffin, Xanthan Gum
- PRINCIPAL DISPLAY PANEL
- MAG 5 PT JAR LABEL AND BOX
-
INGREDIENTS AND APPEARANCE
MAG 5 PT WARMING FORMULA
menthol creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76348-511 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 5.6 g in 112 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) OLIVE OIL (UNII: 6UYK2W1W1E) PEG-100 STEARATE (UNII: YD01N1999R) WITCH HAZEL (UNII: 101I4J0U34) SHEA BUTTER (UNII: K49155WL9Y) LAURETH-7 (UNII: Z95S6G8201) PEPPERMINT OIL (UNII: AV092KU4JH) HIPPOPHAE RHAMNOIDES SEED OIL (UNII: T53SBG6741) C13-14 ISOPARAFFIN (UNII: E4F12ROE70) WHITE WAX (UNII: 7G1J5DA97F) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) LOBELIA INFLATA LEAF (UNII: 7QFT17RLRG) STEARIC ACID (UNII: 4ELV7Z65AP) COCONUT OIL (UNII: Q9L0O73W7L) MAGNESIUM MYRISTATE (UNII: Z1917F0578) TABASCO PEPPER (UNII: J1M3NA843L) MANGIFERA INDICA SEED BUTTER (UNII: 4OXD9M35X2) NIACINAMIDE (UNII: 25X51I8RD4) CANNABIS SATIVA SEED OIL (UNII: 69VJ1LPN1S) CORN OIL (UNII: 8470G57WFM) MAGNESIUM OROTATE (UNII: GI96W46M5A) CANNABIDIOL (UNII: 19GBJ60SN5) MAGNESIUM GLUCONATE (UNII: T42NAD2KHC) HIPPOPHAE RHAMNOIDES FRUIT OIL (UNII: TA4JCF9S1J) CETEARYL GLUCOSIDE (UNII: 09FUA47KNA) PHENOXYETHANOL (UNII: HIE492ZZ3T) MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) POLYACRYLAMIDE (10000 MW) (UNII: E2KR9C9V2I) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76348-511-05 1 in 1 BOX 03/19/2019 1 NDC:76348-511-04 112 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 03/19/2019 Labeler - RENU LABORATORIES, INC. (945739449) Establishment Name Address ID/FEI Business Operations RENU LABORATORIES, INC. 945739449 manufacture(76348-511)