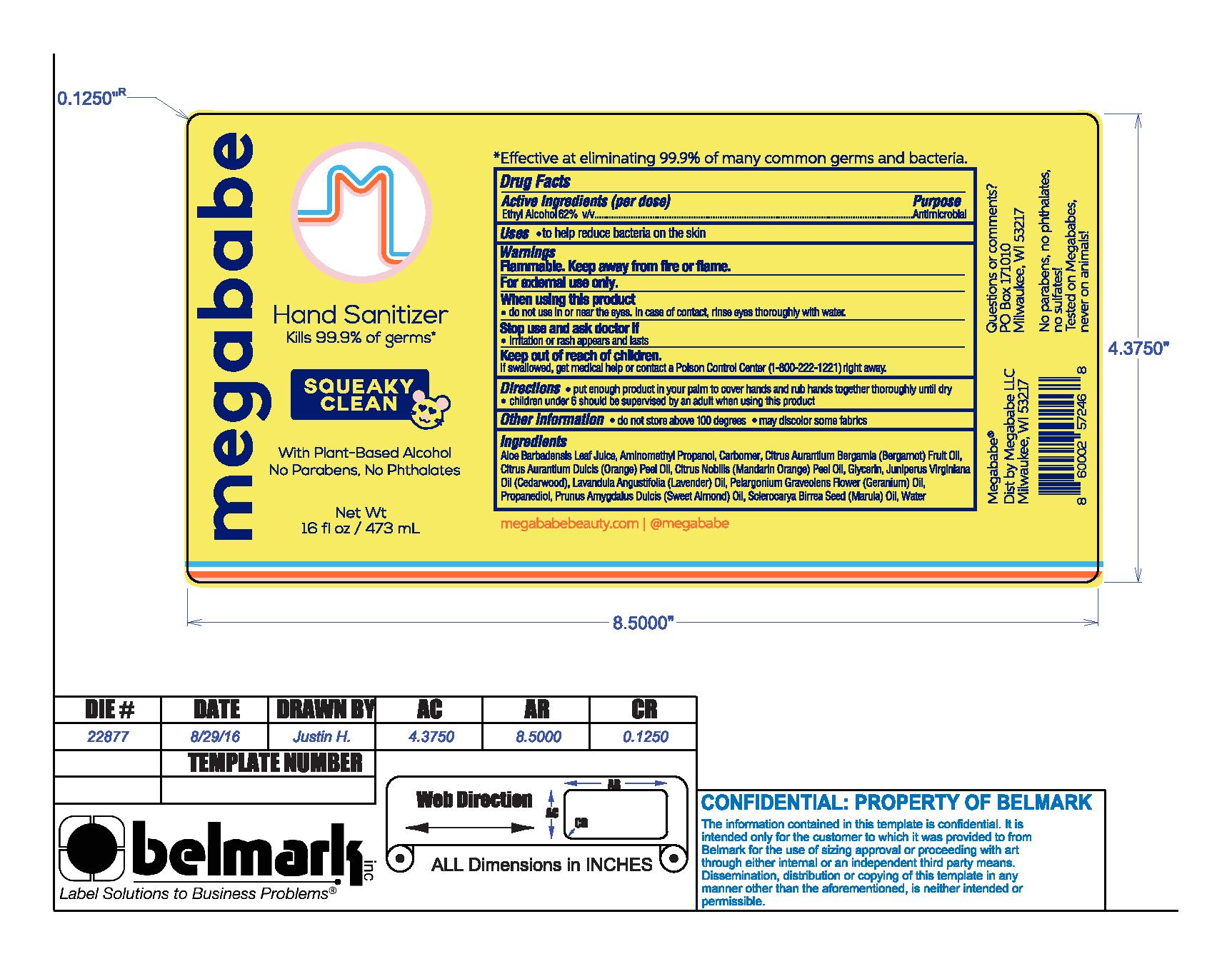
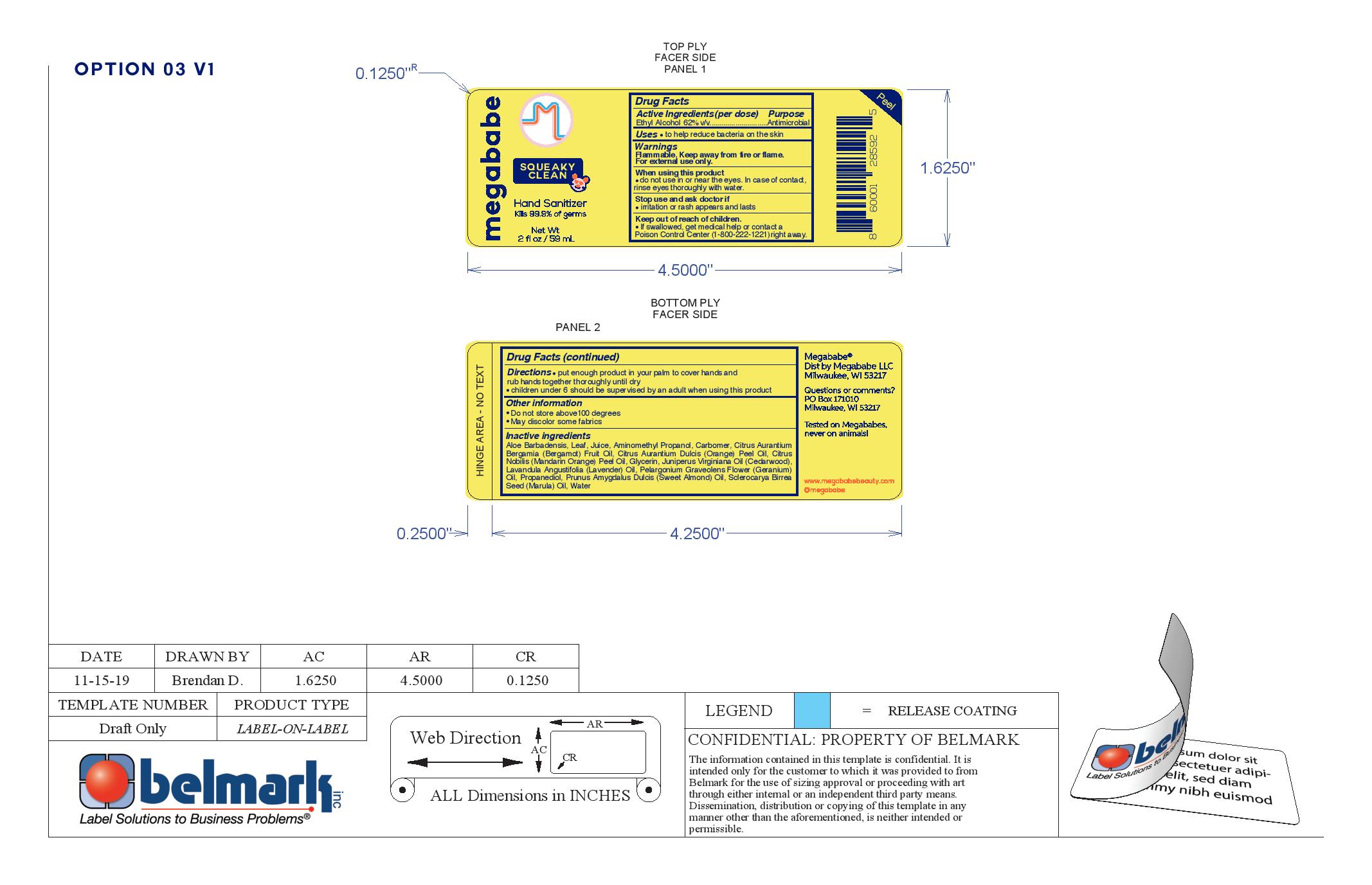
Label: SQUEEKY CLEAN- hand sanitizer liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 76150-243-44, 76150-243-45, 76150-243-46 - Packager: Bell International Laboratories, Inc.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 28, 2020
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Uses
- Warnings
- Directions
- Other Information
-
Ingredients
Aloe Barbadensis Leaf Juice, Aminomethyl Propanol, Carbomer, Citrus Aruantium Bergamia (Bergamot) Fruit Oil, Citrus Aurantium Dulcis (Orange) Peel Oil, Citrus Nobilis (Mandarin Orange) Peel Oil, Glycerin, Juniperus Virginiana Oil (Cedarwood), Lavandula Angustifolia (Lavender) Oil, Pelargonium Graveolens Flower (Geranium) Oil, Propanediol, Prunus Amygdalus Dulcis (sweet almond) Oil,, Sclerocarya Birrea Seed (Marula) Oil, Water
- Product labels
-
INGREDIENTS AND APPEARANCE
SQUEEKY CLEAN
hand sanitizer liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76150-243 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 62 mL in 100 mL Inactive Ingredients Ingredient Name Strength MANDARIN OIL (UNII: NJO720F72R) ALOE VERA LEAF (UNII: ZY81Z83H0X) WATER (UNII: 059QF0KO0R) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) PROPANEDIOL (UNII: 5965N8W85T) GLYCERIN (UNII: PDC6A3C0OX) ORANGE OIL (UNII: AKN3KSD11B) JUNIPERUS VIRGINIANA OIL (UNII: PAD4FN7P2G) SCLEROCARYA BIRREA SEED OIL (UNII: WDO4TLS35F) LAVENDER OIL (UNII: ZBP1YXW0H8) PELARGONIUM GRAVEOLENS FLOWER OIL (UNII: 3K0J1S7QGC) ALMOND OIL (UNII: 66YXD4DKO9) BERGAMOT OIL (UNII: 39W1PKE3JI) AMINOMETHYLPROPANOL (UNII: LU49E6626Q) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76150-243-44 59 mL in 1 BOTTLE; Type 0: Not a Combination Product 11/01/2019 2 NDC:76150-243-45 237 mL in 1 BOTTLE; Type 0: Not a Combination Product 11/01/2019 3 NDC:76150-243-46 473 mL in 1 BOTTLE; Type 0: Not a Combination Product 11/01/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 11/01/2019 Labeler - Bell International Laboratories, Inc. (967781555)