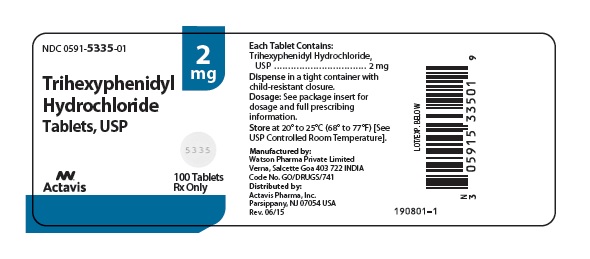
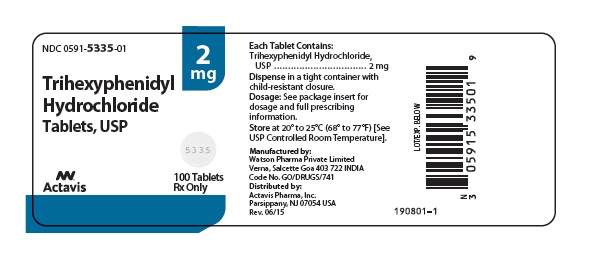
Label: TRIHEXYPHENIDYL HYDROCHLORIDE tablet
- NDC Code(s): 0591-5335-01, 0591-5335-10, 0591-5337-01, 0591-5337-10
- Packager: Actavis Pharma, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 2, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Trihexyphenidyl hydrochloride is a synthetic antispasmodic drug available in the following forms: Tablets, 2 mg and 5 mg. It is designated chemically 1-Piperidinepropanol,α-cyclohexyl-α-phenyl-, hydrochloride. The structural formula is represented below:
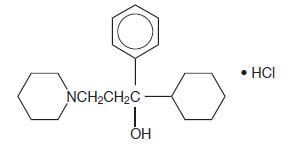
C20H31NO•HCl M.W. 337.93
Trihexyphenidyl hydrochloride tablets USP, 2 mg and 5 mg contain the following inactive ingredients: colloidal silicon dioxide, dibasic calcium phosphate, lactose monohydrate, magnesium stearate, microcrystalline cellulose and sodium starch glycolate.
-
CLINICAL PHARMACOLOGY
Trihexyphenidyl HCl is the substituted piperidine salt, 3-(1-piperidyl)-1-phenylcyclohexyl-1-propanol hydrochloride, which exerts a direct inhibitory effect upon the parasympathetic nervous system. It also has a relaxing effect on smooth musculature; exerted both directly upon the muscle tissue itself and indirectly through an inhibitory effect upon the parasympathetic nervous system. Its therapeutic properties are similar to those of atropine, although undesirable side effects are ordinarily less frequent and severe than with the latter.
-
INDICATIONS
This drug is indicated as an adjunct in the treatment of all forms of parkinsonism (postencephalitic, arteriosclerotic, and idiopathic). It is often useful as adjuvant therapy when treating these forms of parkinsonism with levodopa. Additionally, it is indicated for the control of extrapyramidal disorders caused by central nervous system drugs such as the dibenzoxazepines, phenothiazines, thioxanthenes, and butyrophenones.
- WARNINGS
-
PRECAUTIONS
Although trihexyphenidyl HCl is not contraindicated for patients with cardiac, liver, or kidney disorders, or with hypertension, such patients should be maintained under close observation.
Since the use of trihexyphenidyl HCl may, in some cases, continue indefinitely and since it has atropine-like properties, patients should be subjected to constant and careful long-term observation to avoid allergic and other untoward reactions. In as much as trihexyphenidyl HCl possesses some parasympatholytic activity, it should be used with caution in patients with glaucoma, obstructive disease of the gastrointestinal or genitourinary tracts, and in elderly males with possible prostatic hypertrophy. Geriatric patients, particularly over the age of 60, frequently develop increased sensitivity to the actions of drugs of this type, and hence, require strict dosage regulation. Incipient glaucoma may be precipitated by parasympatholytic drugs such as trihexyphenidyl HCl.
Tardive dyskinesia may appear in some patients on long term therapy with antipsychotic drugs or may occur after therapy when these drugs have been discontinued. Antiparkinson agents do not alleviate the symptoms of tardive dyskinesia and in some instances may aggravate them. However, parkinsonism and tardive dyskinesia often coexist in patients receiving chronic neuroleptic treatment, and anticholinergic therapy with trihexyphenidyl hydrochloride may relieve some of these parkinsonism symptoms.
-
ADVERSE REACTIONS
Minor side effects, such as dryness of the mouth, blurring of vision, dizziness, mild nausea, or nervousness, will be experienced by 30 to 50 percent of all patients. These sensations, however, are much less troublesome with trihexyphenidyl HCl than with belladonna alkaloids and are usually less disturbing than unalleviated parkinsonism. Such reactions tend to become less pronounced, and even to disappear, as treatment continues. Even before these reactions have remitted spontaneously, they may often be controlled by careful adjustment of the dosage form, amount of drug, or interval between doses.
Isolated instances of suppurative parotitis secondary to excessive dryness of the mouth, skin rashes, dilatation of the colon, paralytic ileus, and certain psychiatric manifestations such as delusions and hallucinations, plus one doubtful case of paranoia all of which may occur with any of the atropine-like drugs, have been rarely reported with trihexyphenidyl hydrochloride.
Patients with arteriosclerosis or with a history of idiosyncrasy to other drugs may exhibit reactions of mental confusion, agitation, disturbed behavior, or nausea and vomiting. Such patients should be allowed to develop a tolerance through the initial administration of a small dose and gradual increase in dose until an effective level is reached. If a severe reaction should occur, administration of the drug should be discontinued for a few days and then resumed at a lower dosage. Psychiatric disturbances can result from indiscriminate use (leading to overdosage) to sustain continued euphoria.
Potential side effects associated with the use of any atropine-like drugs include constipation, drowsiness, urinary hesitancy or retention, tachycardia, dilation of the pupil, increased intraocular tension, weakness, vomiting, and headache.
The occurrence of angle-closure glaucoma due to long-term treatment with trihexyphenidyl hydrochloride has been reported.
To report SUSPECTED ADVERSE EVENTS, contact Teva at 1-888-838-2872 or FDA at 1-800-FDA-1088 or http://www.fda.gov/medwatch for voluntary reporting of adverse reactions.
-
DOSAGE AND ADMINISTRATION
Dosage should be individualized. The initial dose should be low and then increased gradually, especially in patients over 60 years of age. Whether trihexyphenidyl HCl may best be given before or after meals should be determined by the way the patient reacts. Postencephalitic patients, who are usually more prone to excessive salivation, may prefer to take it after meals and may, in addition, require small amounts of atropine which, under such circumstances, is sometimes an effective adjuvant. If trihexyphenidyl HCl tends to dry the mouth excessively, it may be better to take it before meals, unless it causes nausea. If taken after meals, the thirst sometimes induced can be allayed by mint candies, chewing gum or water.
Trihexyphenidyl HCl in Idiopathic Parkinsonism
As initial therapy for parkinsonism, 1 mg may be administered the first day. The dose may then be increased by 2 mg increments at intervals of three to five days, until a total of 6 to 10 mg is given daily. The total daily dose will depend upon what is found to be the optimal level. Many patients derive maximum benefit from this daily total of 6 to 10 mg, but some patients, chiefly those in the postencephalitic group, may require a total daily dose of 12 to 15 mg.
Trihexyphenidyl HCl in Drug-Induced Parkinsonism
The size and frequency of dose of trihexyphenidyl HCl needed to control extrapyramidal reactions to commonly employed tranquilizers, notably the phenothiazines, thioxanthenes, and butyrophenones, must be determined empirically. The total daily dosage usually ranges between 5 and 15 mg, although, in some cases, these reactions have been satisfactorily controlled on as little as 1 mg daily. It may be advisable to commence therapy with a single 1 mg dose. If the extrapyramidal manifestations are not controlled in a few hours, the subsequent doses may be progressively increased until satisfactory control is achieved. Satisfactory control may sometimes be more rapidly achieved by temporarily reducing the dosage of the tranquilizer on instituting trihexyphenidyl HCl therapy and then adjusting dosage of both drugs until the desired ataractic effect is retained without onset of extrapyramidal reactions.
It is sometimes possible to maintain the patient on a reduced trihexyphenidyl HCl dosage after the reactions have remained under control for several days. Instances have been reported in which these reactions have remained in remission for long periods after trihexyphenidyl HCl therapy was discontinued.
Concomitant Use of Trihexyphenidyl HCl with Levodopa
When trihexyphenidyl HCl is used concomitantly with levodopa, the usual dose of each may need to be reduced. Careful adjustment is necessary, depending on side effects and degree of symptom control. Trihexyphenidyl HCl dosage of 3 to 6 mg daily, in divided doses, is usually adequate.
Concomitant Use of Trihexyphenidyl HCl with Other Parasympathetic Inhibitors
Trihexyphenidyl HCl may be substituted, in whole or in part, for other parasympathetic inhibitors. The usual technique is partial substitution initially, with progressive reduction in the other medication as the dose of trihexyphenidyl HCl is increased.
Trihexyphenidyl Hydrochloride Tablets - The total daily intake of trihexyphenidyl HCl tablets is tolerated best if divided into 3 doses and taken at mealtimes. High doses (> 10 mg daily) may be divided into 4 parts, with 3 doses administered at meal times and the fourth at bedtime.
-
HOW SUPPLIED
Trihexyphenidyl hydrochloride tablets USP, 2 mg are scored, round, white, flat faced, tablets imprinted DAN DAN and 5335 supplied in bottles of 100 (NDC 0591-5335-01) and 1000 (NDC 0591-5335-10).
Trihexyphenidyl hydrochloride tablets USP, 5 mg are scored, round, white, tablets imprinted DAN DAN and 5337 supplied in bottles of 100 (NDC 0591-5337-01) and 1000 (NDC 0591-5337-10).
Dispense in a tight container with child-resistant closure.
Store at 20° to 25° (68° to 77°F) [See USP Controlled Room Temperature].
Manufactured In India By:
Watson Pharma Private Limited
Verna, Salcette Goa 403 722 INDIAManufactured For:
Teva Pharmaceuticals
Parsippany, NJ 07054Rev. A 2/2024
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
TRIHEXYPHENIDYL HYDROCHLORIDE
trihexyphenidyl hydrochloride tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0591-5335 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TRIHEXYPHENIDYL HYDROCHLORIDE (UNII: AO61G82577) (TRIHEXYPHENIDYL - UNII:6RC5V8B7PO) TRIHEXYPHENIDYL HYDROCHLORIDE 2 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) Product Characteristics Color white Score 2 pieces Shape ROUND (flat faced) Size 8mm Flavor Imprint Code DAN;DAN;5335 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0591-5335-01 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/01/1987 2 NDC:0591-5335-10 1000 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/01/1987 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA084363 11/01/1987 TRIHEXYPHENIDYL HYDROCHLORIDE
trihexyphenidyl hydrochloride tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0591-5337 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TRIHEXYPHENIDYL HYDROCHLORIDE (UNII: AO61G82577) (TRIHEXYPHENIDYL - UNII:6RC5V8B7PO) TRIHEXYPHENIDYL HYDROCHLORIDE 5 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) Product Characteristics Color white Score 2 pieces Shape ROUND Size 10mm Flavor Imprint Code DAN;DAN;5337 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0591-5337-01 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/01/1987 2 NDC:0591-5337-10 1000 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/01/1987 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA084364 11/01/1987 Labeler - Actavis Pharma, Inc. (119723554)