Label: RX-SPECIMEN COLLECTION KIT- specimen collection kit kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 10819-3740-1, 33358-0149-1, 69677-030-01 - Packager: MAS Management Group Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 22, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- WARNINGS AND PRECAUTIONS
- COMPONENTS
- KEEP OUT OF REACH OF CHILDREN
- DIAGRAM OF DEVICE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
RX-SPECIMEN COLLECTION KIT
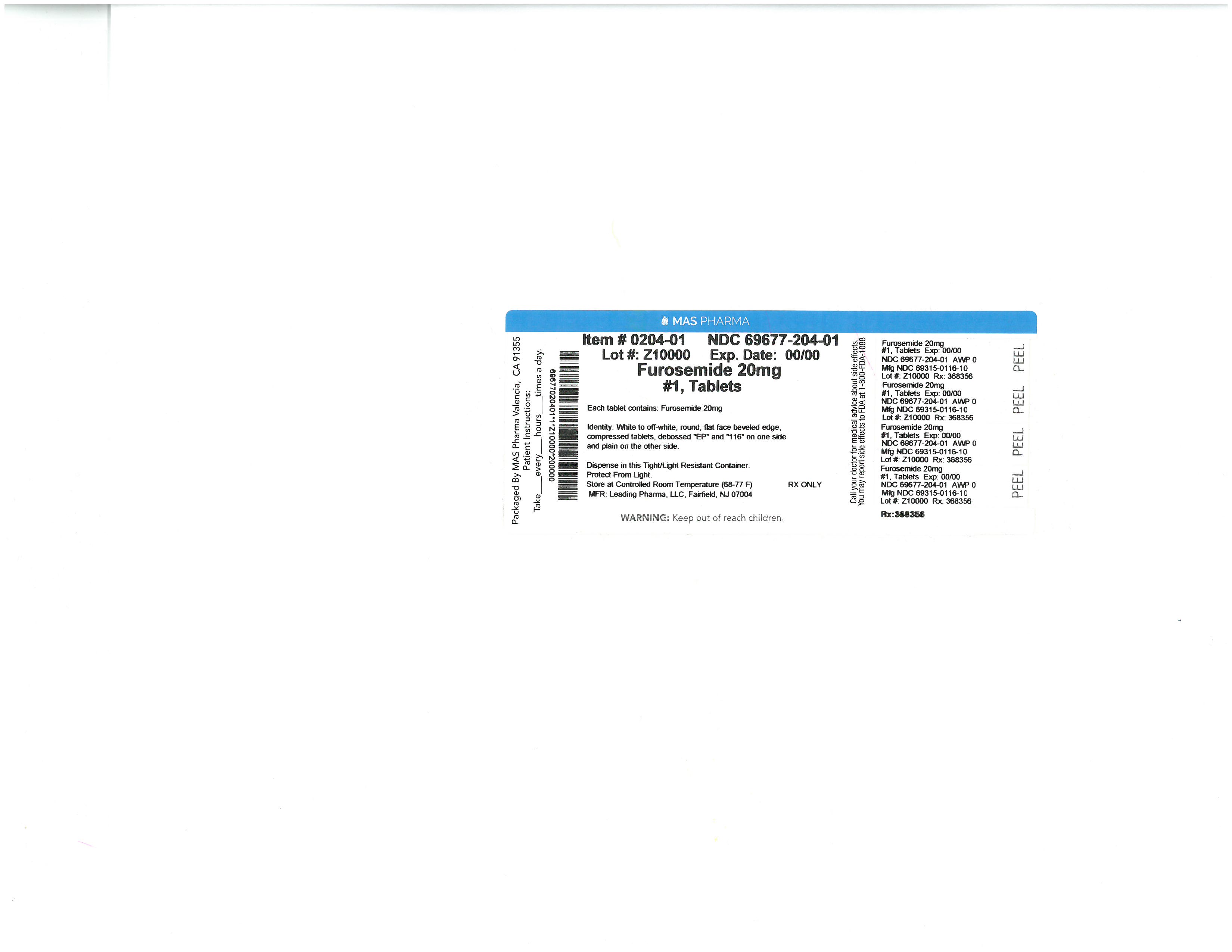
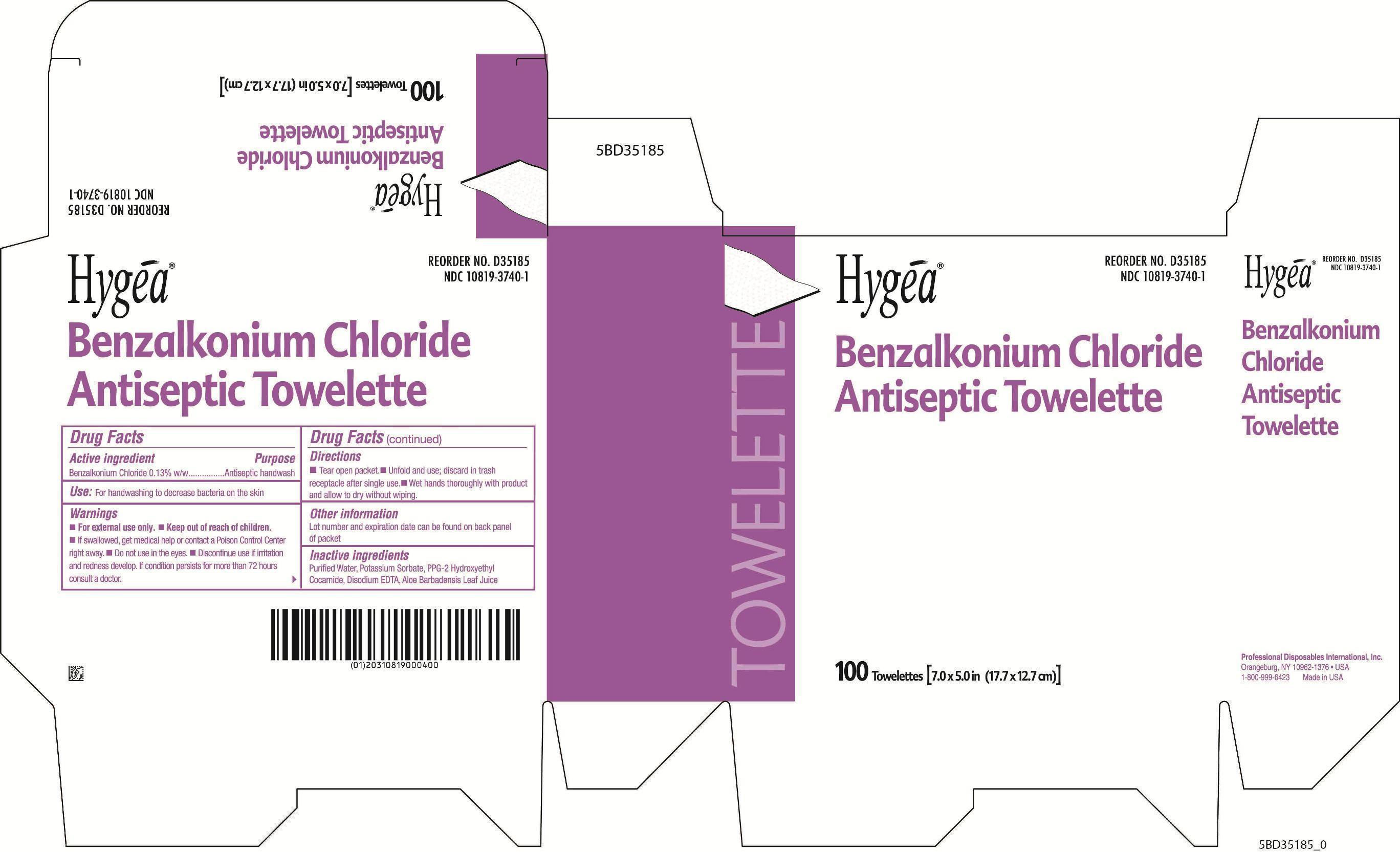
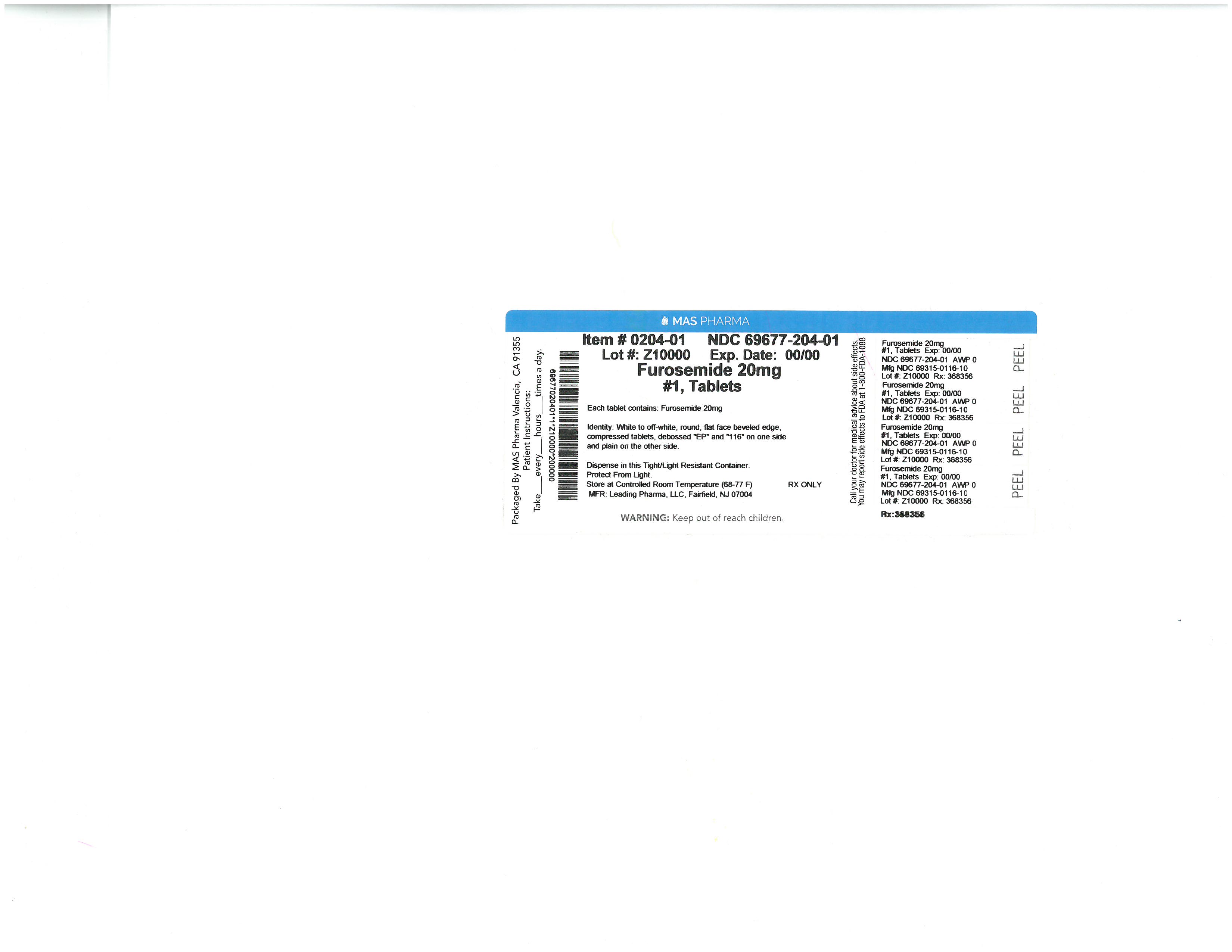
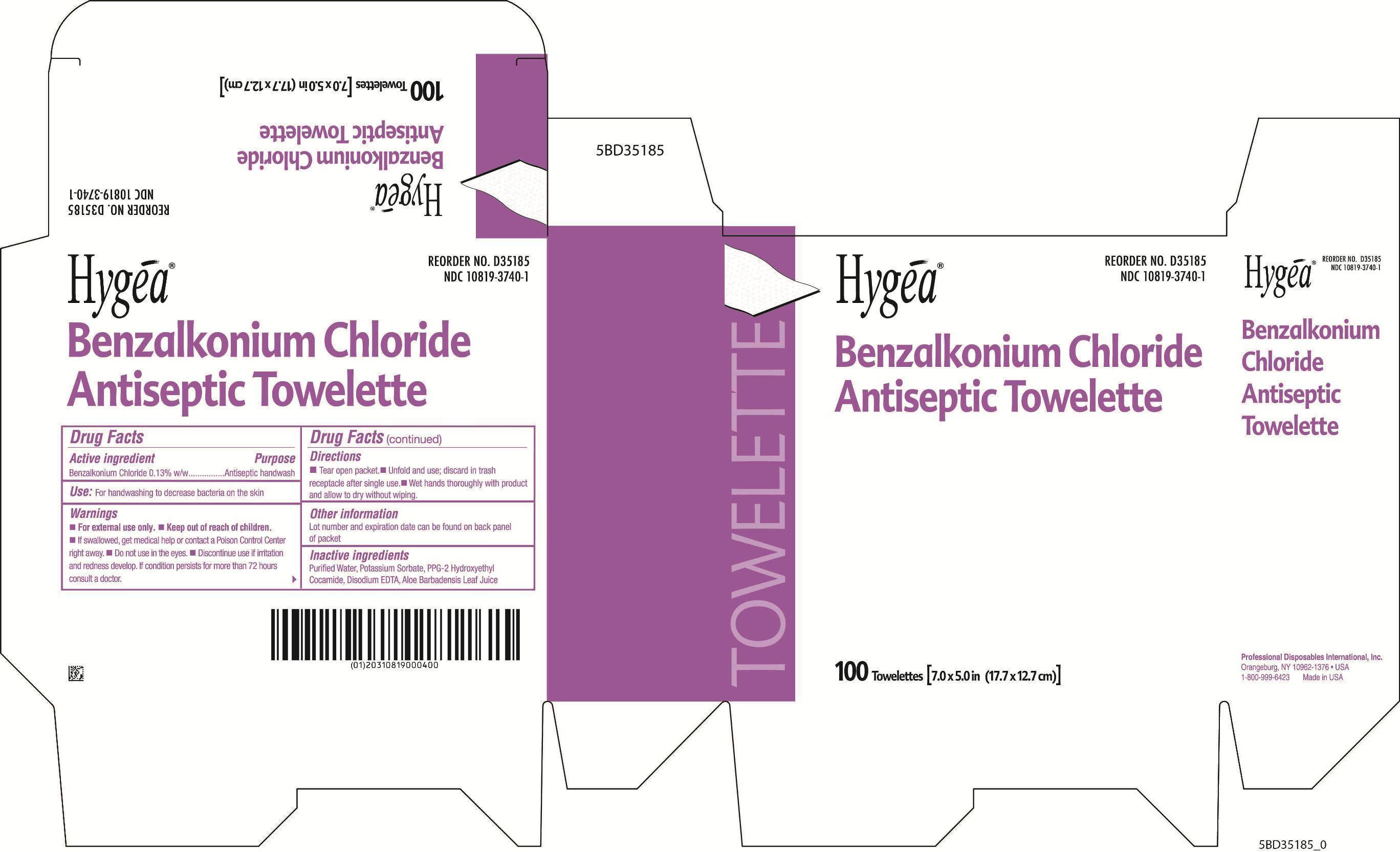
specimen collection kit kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:69677-030 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69677-030-01 1 in 1 PACKAGE; Type 1: Convenience Kit of Co-Package 07/23/2015 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 PACKAGE 1 Part 2 1 PACKET 3 mL Part 1 of 2 FUROSEMIDE
furosemide pillProduct Information Item Code (Source) NDC:33358-0149 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FUROSEMIDE (UNII: 7LXU5N7ZO5) (FUROSEMIDE - UNII:7LXU5N7ZO5) FUROSEMIDE 20 mg Inactive Ingredients Ingredient Name Strength MODIFIED CORN STARCH (1-OCTENYL SUCCINIC ANHYDRIDE) (UNII: 461P5CJN6T) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) STARCH, PREGELATINIZED CORN (UNII: O8232NY3SJ) SODIUM STARCH GLYCOLATE TYPE A CORN (UNII: AG9B65PV6B) Product Characteristics Color white Score no score Shape ROUND Size 6mm Flavor Imprint Code RE22 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:33358-0149-1 1 in 1 PACKAGE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078010 07/23/2015 Part 2 of 2 BENZALKONIUM CHLORIDE
benzalkonium chloride swabProduct Information Item Code (Source) NDC:10819-3740 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.0013 g in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) PPG-2 HYDROXYETHYL COCAMIDE (UNII: 34N07GUJ3X) EDETATE DISODIUM (UNII: 7FLD91C86K) ALOE VERA LEAF (UNII: ZY81Z83H0X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10819-3740-1 3 mL in 1 PACKET; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 07/23/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078010 07/23/2015 Labeler - MAS Management Group Inc. (079363782) Establishment Name Address ID/FEI Business Operations MAS Management Group Inc. 079363782 manufacture(69677-030) Establishment Name Address ID/FEI Business Operations RxChange Co 781126805 manufacture(33358-0149) Establishment Name Address ID/FEI Business Operations Professional Disposable International, Inc 800777117 manufacture(10819-3740)