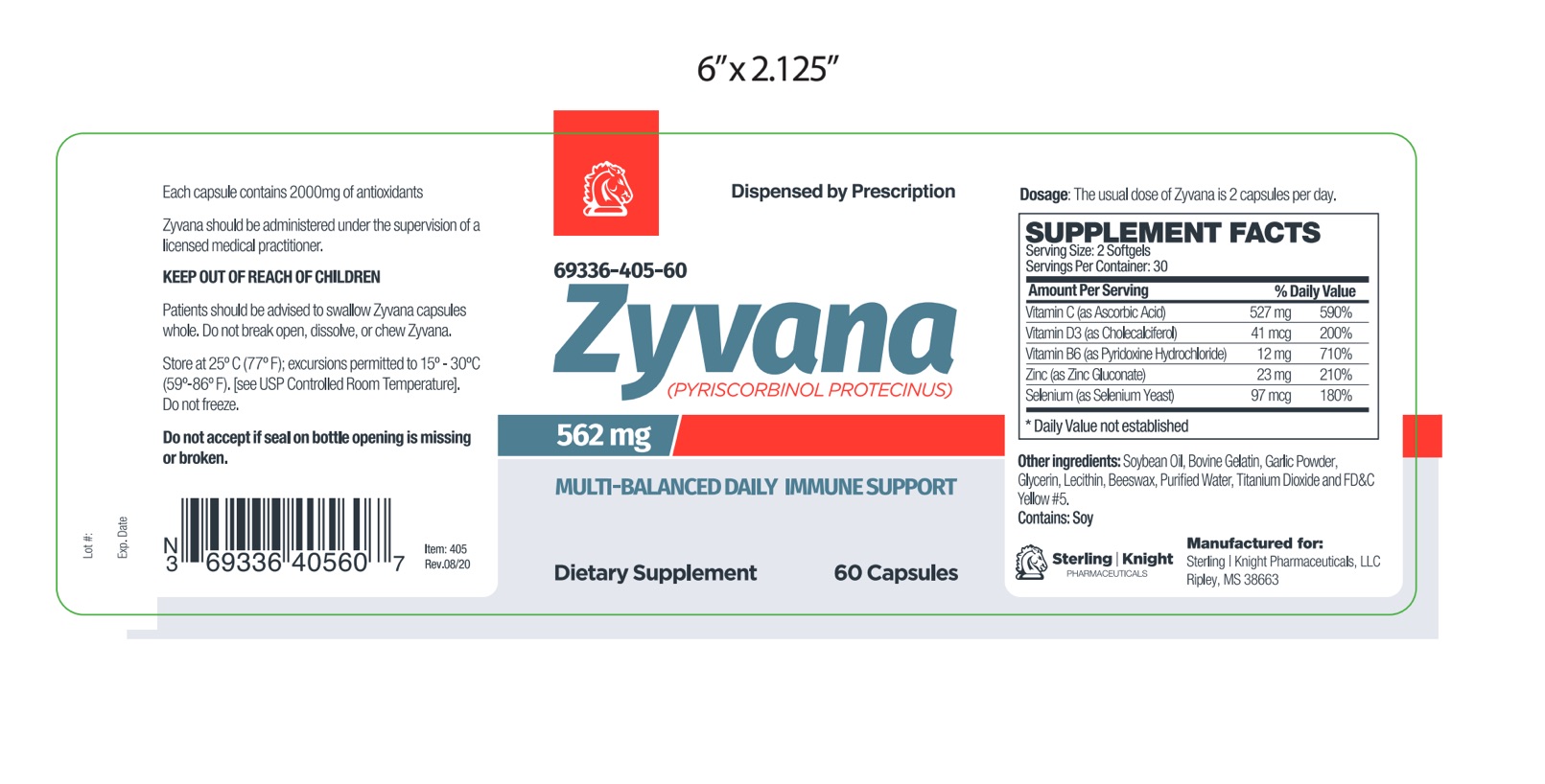
Label: ZYVANA capsule
-
Contains inactivated NDC Code(s)
NDC Code(s): 69336-405-60 - Packager: Sterling-Knight Pharmaceuticals, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated February 22, 2021
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Zyvana is an orally administered prescription dietary supplement formulation.
Zyvana should be administered under the supervision of a licensed medical practitioner.
Other Ingredients: Soybean Oil, Gelatin (bovine), Garlic Powder, Glycerin, Lecithin, Beeswax, Purified Water, Titanium Dioxide, FD&C Yellow #5.
- INDICATIONS AND USAGE
-
WARNINGS AND PRECAUTIONS:
This product is contraindicated in patients with a known hypersensitivity to any of the ingredients.Zyvana should only be used under the direction and supervision of a licensed medical practitioner. Use with caution in patients that may have a medical condition, are pregnant, lactating, trying to conceive, under the age of 18, or taking medications.
- DOSAGE AND ADMINISTRATION
- HOW SUPPLIED SECTION
- STORAGE
- Reserved for Professional Recommendation
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ZYVANA
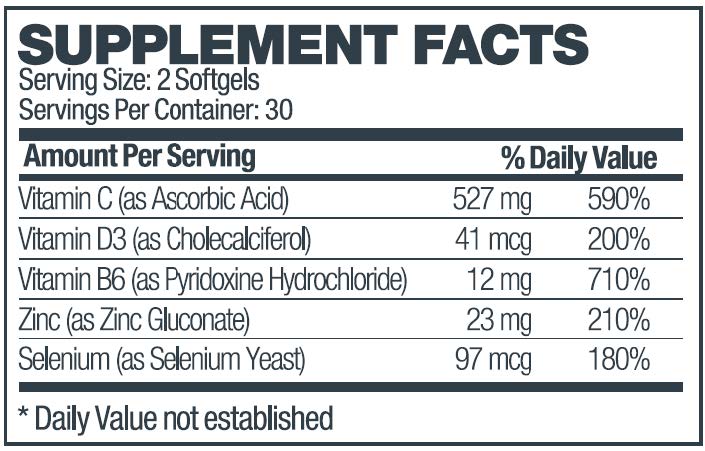
zyvana capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:69336-405 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 527 mg CHOLECALCIFEROL (UNII: 1C6V77QF41) (CHOLECALCIFEROL - UNII:1C6V77QF41) CHOLECALCIFEROL 0.041 mg PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE 12 mg ZINC GLUCONATE (UNII: U6WSN5SQ1Z) (ZINC CATION - UNII:13S1S8SF37) ZINC GLUCONATE 23 mg SELENIUM (UNII: H6241UJ22B) (SELENIUM - UNII:H6241UJ22B) SELENIUM 0.097 mg Inactive Ingredients Ingredient Name Strength SOYBEAN OIL (UNII: 241ATL177A) GELATIN (UNII: 2G86QN327L) GARLIC (UNII: V1V998DC17) GLYCERIN (UNII: PDC6A3C0OX) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) PEG-8 BEESWAX (UNII: 3C1QUF1TIR) WATER (UNII: 059QF0KO0R) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) Product Characteristics Color yellow (softgel capsule) Score 4 pieces Shape capsule Size 14mm Flavor Imprint Code 405 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69336-405-60 60 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 02/22/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 02/22/2021 Labeler - Sterling-Knight Pharmaceuticals, LLC (079556942)