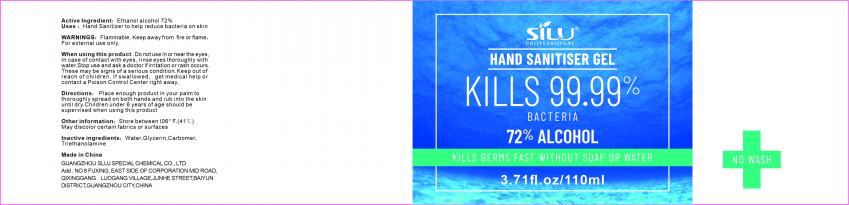
Label: HAND SANITISER GEL gel
-
Contains inactivated NDC Code(s)
NDC Code(s): 75763-001-01, 75763-001-02, 75763-001-03, 75763-001-04, view more75763-001-05, 75763-001-06, 75763-001-07, 75763-001-08, 75763-001-09, 75763-001-10, 75763-001-11, 75763-001-12, 75763-001-13, 75763-001-14, 75763-001-15, 75763-001-16, 75763-001-17, 75763-001-18, 75763-001-19, 75763-001-20 - Packager: GUANGZHOU SLUJ SPECIAL CHEMICAL CO., LTD
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 25, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Uses
- WARNINGS
- DO NOT USE
- When using this product
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other information
- Inactive ingredients
- Package Label - Principal Display Panel
-
INGREDIENTS AND APPEARANCE
HAND SANITISER GEL
hand sanitiser gel gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:75763-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 72 mL in 100 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) TROLAMINE (UNII: 9O3K93S3TK) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75763-001-01 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/25/2020 2 NDC:75763-001-02 50 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/25/2020 3 NDC:75763-001-03 60 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/25/2020 4 NDC:75763-001-04 100 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/25/2020 5 NDC:75763-001-05 110 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/25/2020 6 NDC:75763-001-06 120 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/25/2020 7 NDC:75763-001-07 150 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/25/2020 8 NDC:75763-001-08 180 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/25/2020 9 NDC:75763-001-09 200 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/25/2020 10 NDC:75763-001-10 300 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/25/2020 11 NDC:75763-001-11 350 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/25/2020 12 NDC:75763-001-12 400 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/25/2020 13 NDC:75763-001-13 500 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/25/2020 14 NDC:75763-001-14 600 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/25/2020 15 NDC:75763-001-15 800 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/25/2020 16 NDC:75763-001-16 1000 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/25/2020 17 NDC:75763-001-17 2000 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/25/2020 18 NDC:75763-001-18 3000 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/25/2020 19 NDC:75763-001-19 4000 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/25/2020 20 NDC:75763-001-20 5000 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/25/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 04/25/2020 Labeler - GUANGZHOU SLUJ SPECIAL CHEMICAL CO., LTD (526847687) Registrant - GUANGZHOU SLUJ SPECIAL CHEMICAL CO., LTD (526847687) Establishment Name Address ID/FEI Business Operations GUANGZHOU SLUJ SPECIAL CHEMICAL CO., LTD 526847687 manufacture(75763-001)