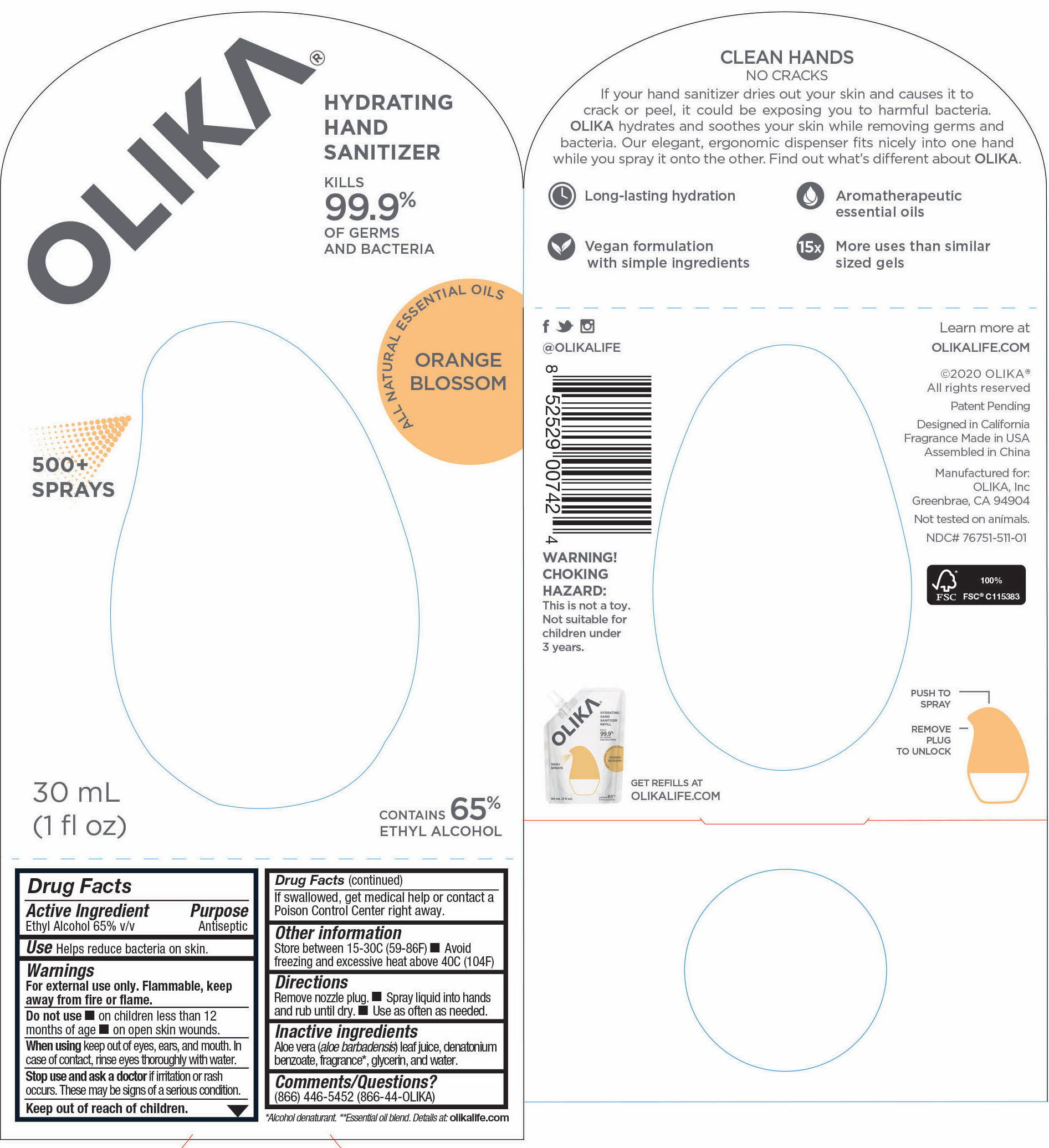
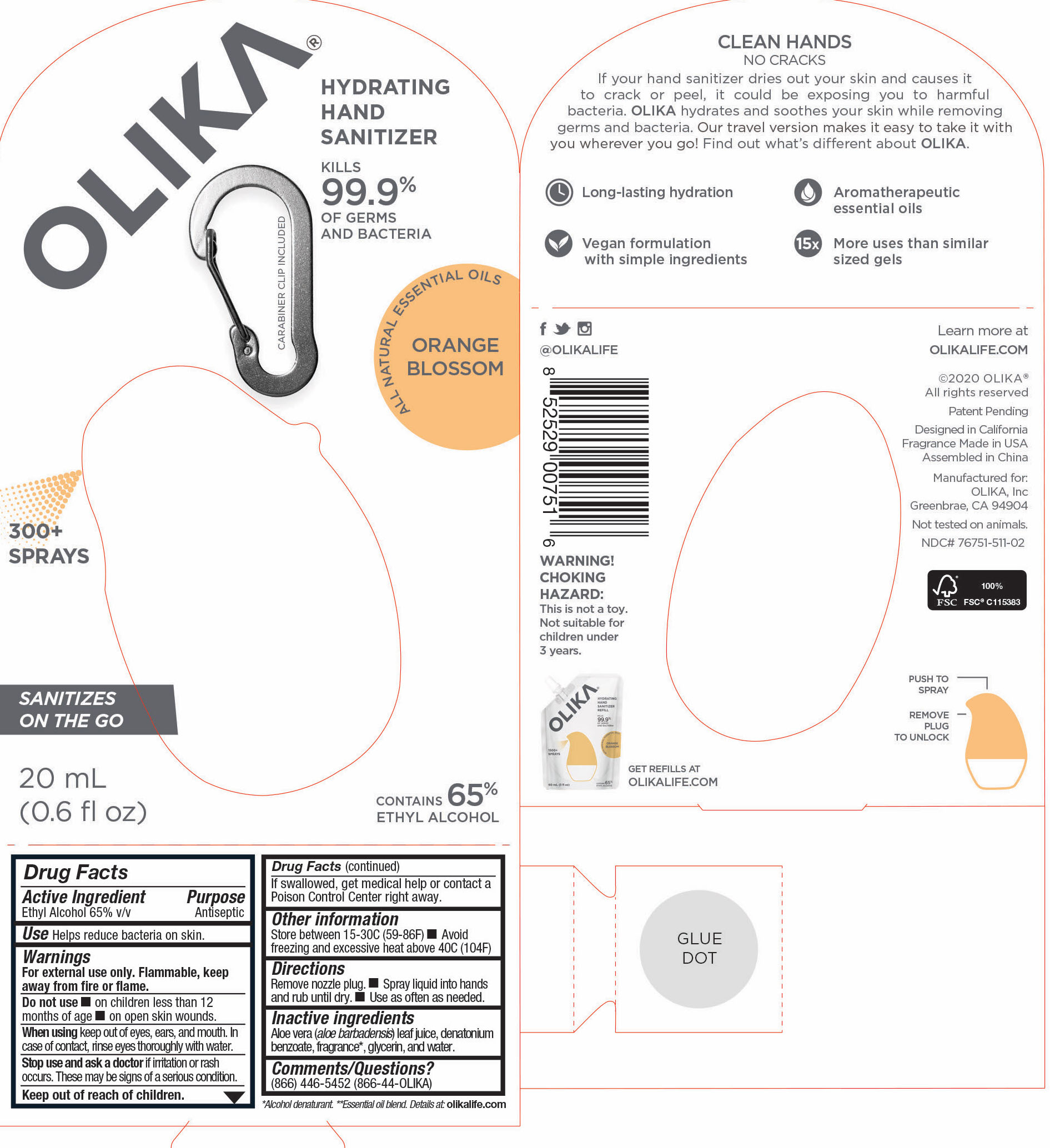
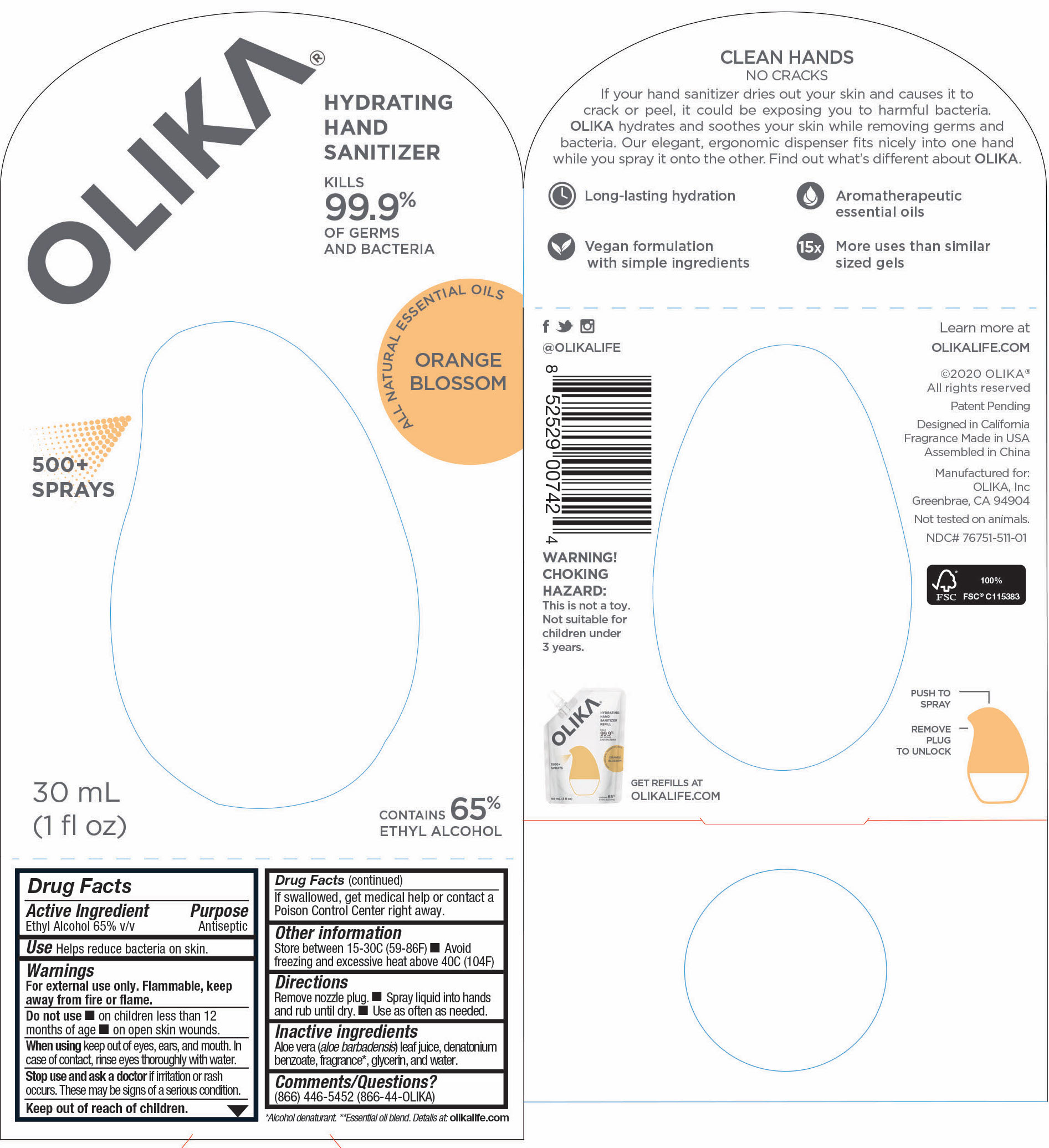
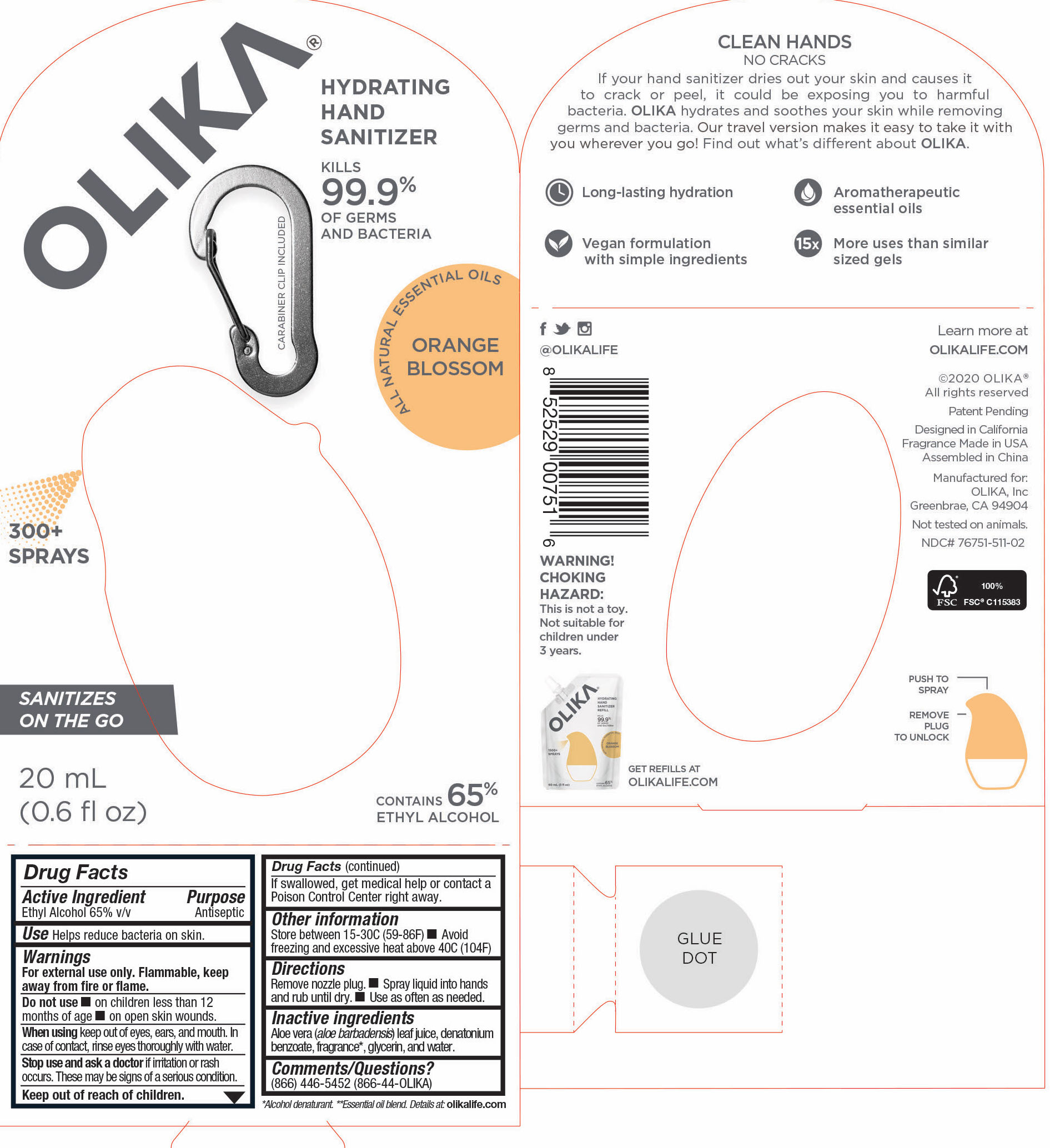
Label: OLIKA HAND SANITIZER ORANGE BLOSSOM- ethyl alcohol liquid
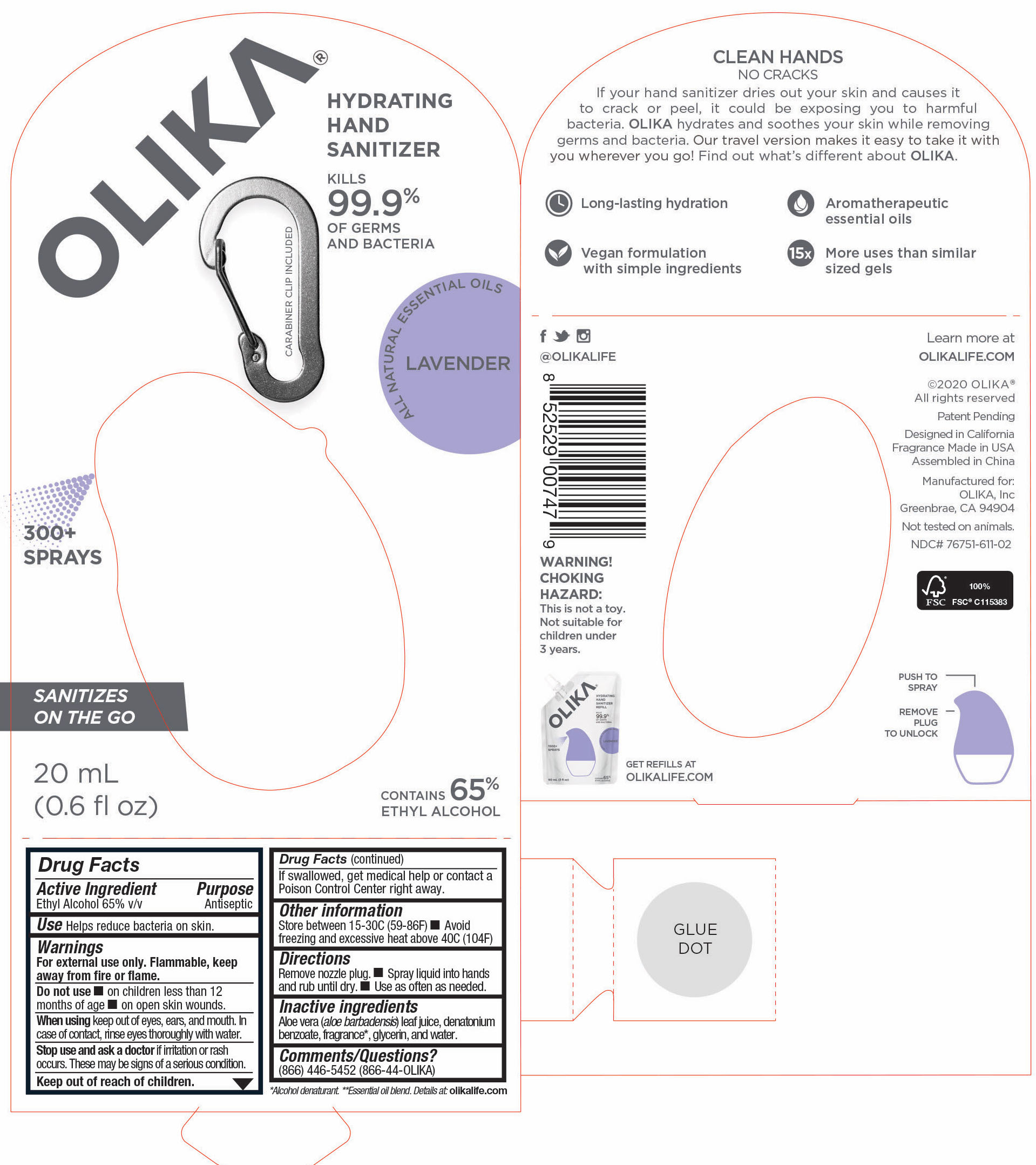
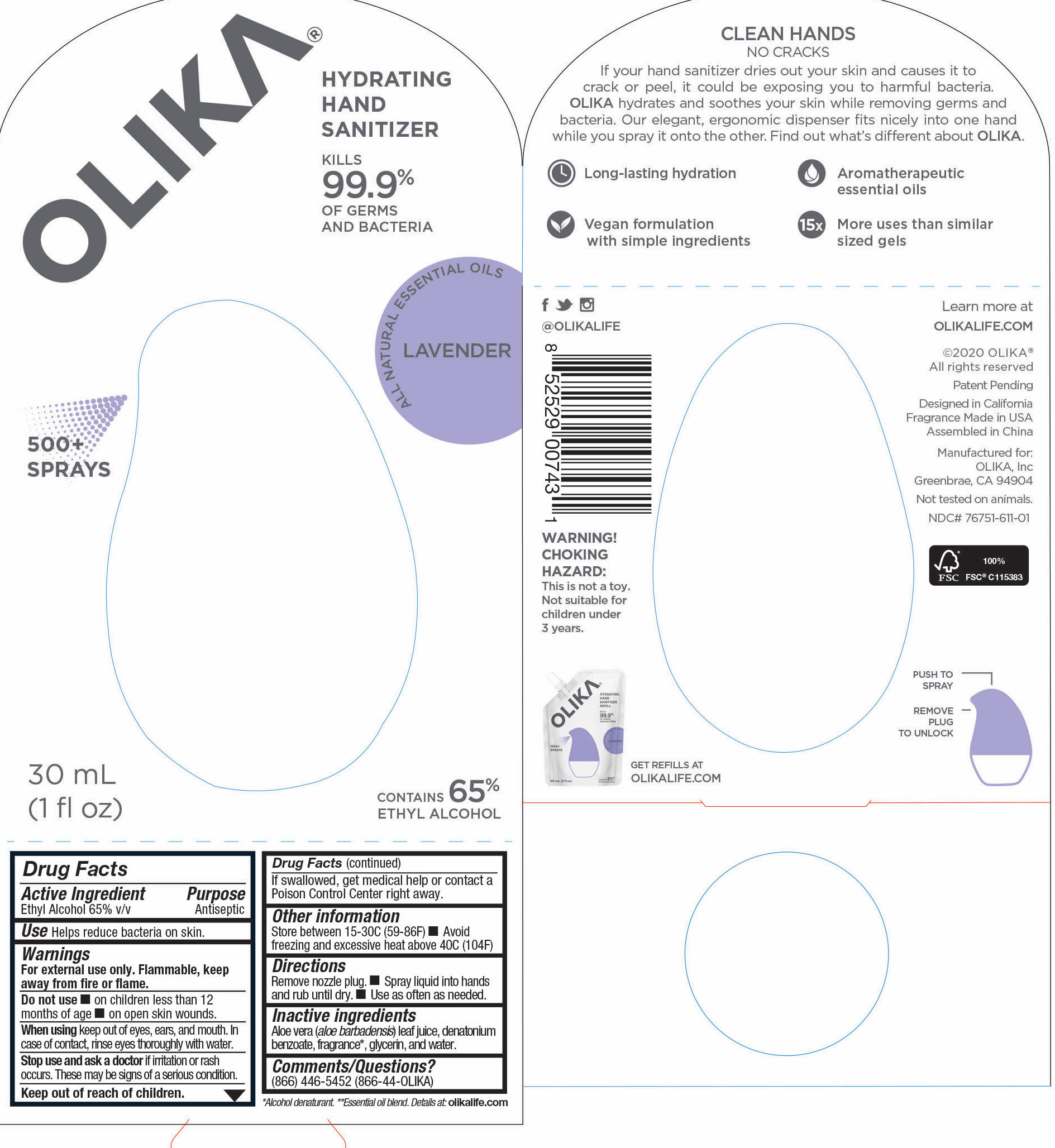
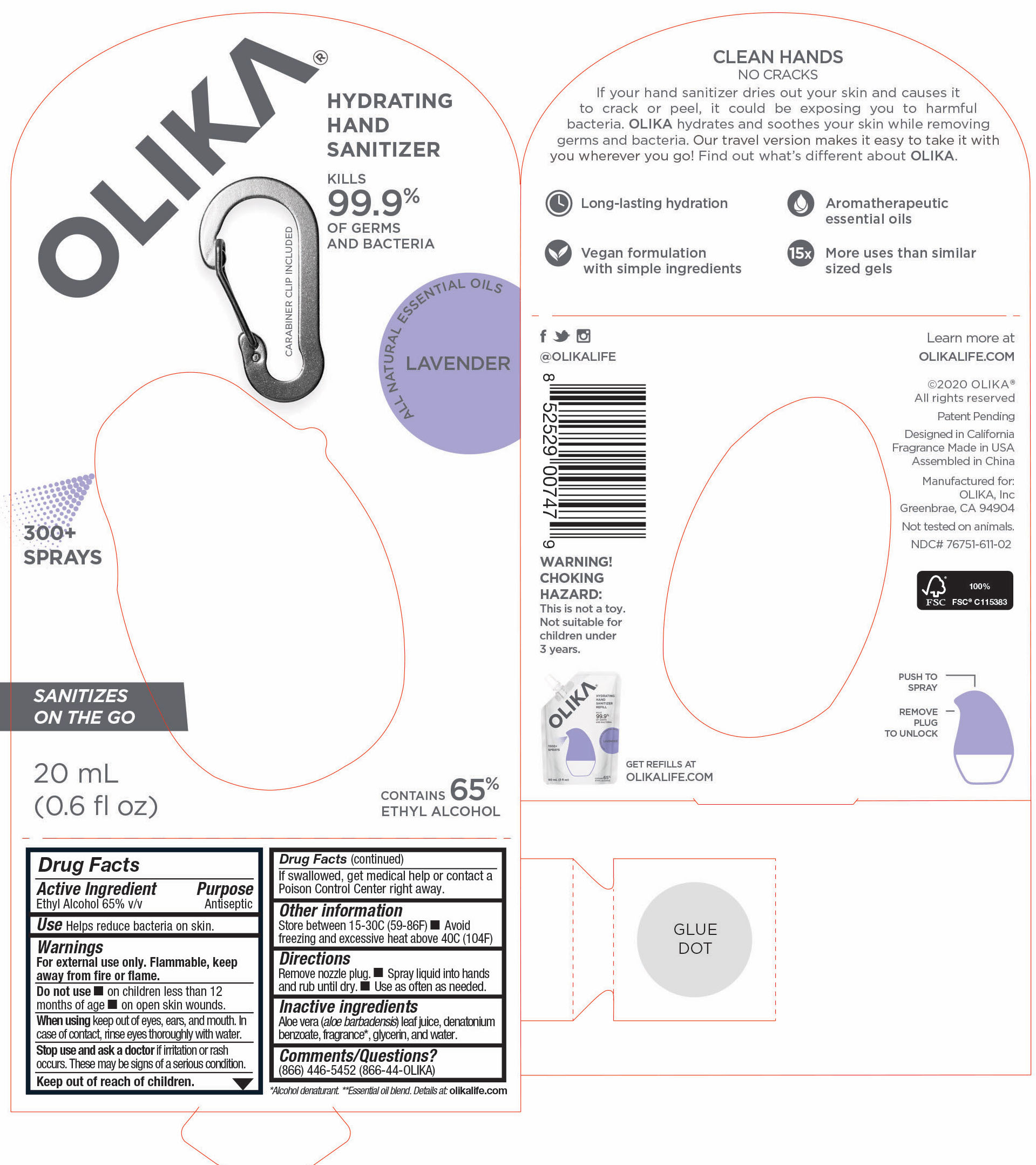
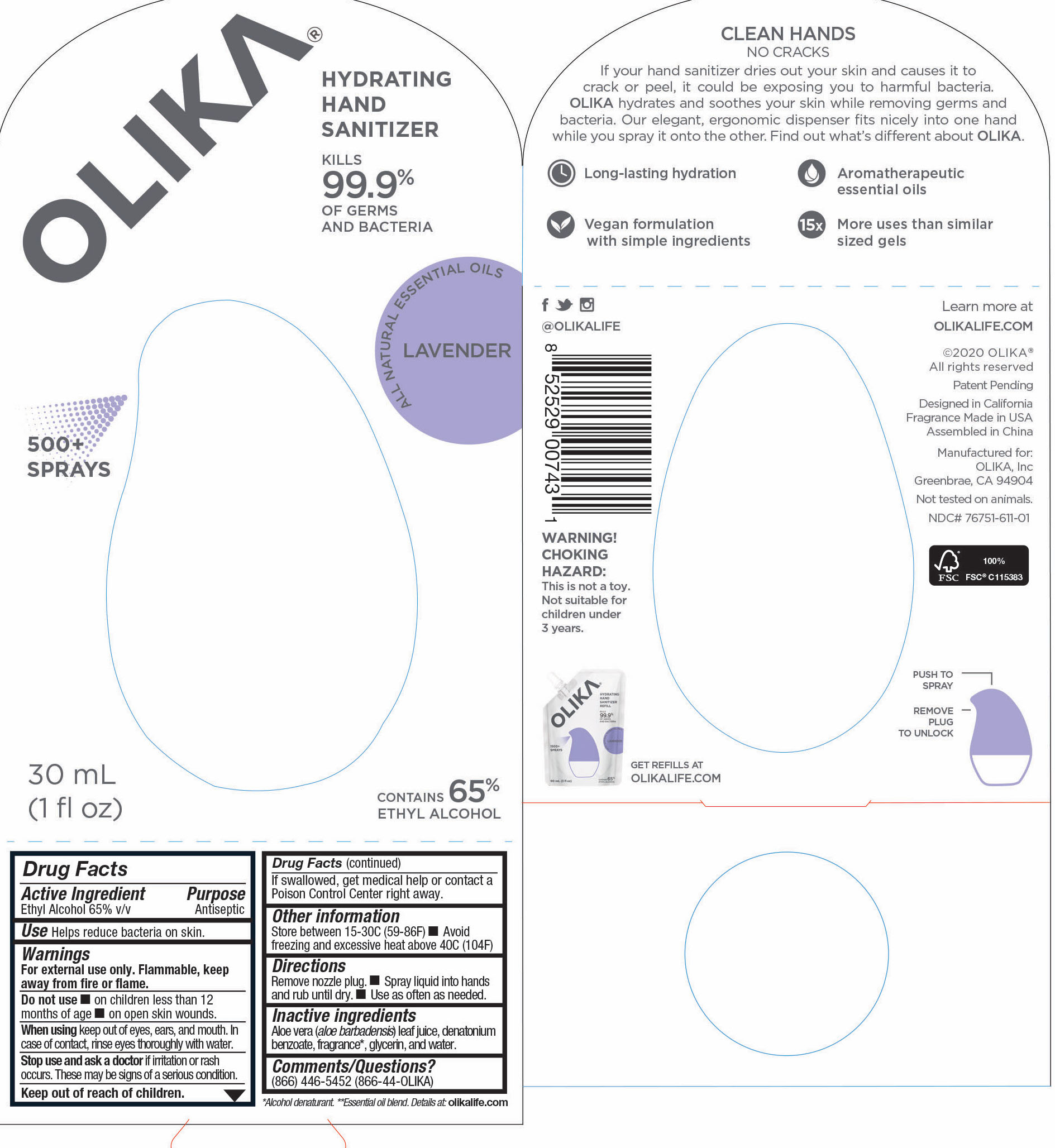
OLIKA HAND SANITIZER LAVENDER- ethyl alcohol liquid
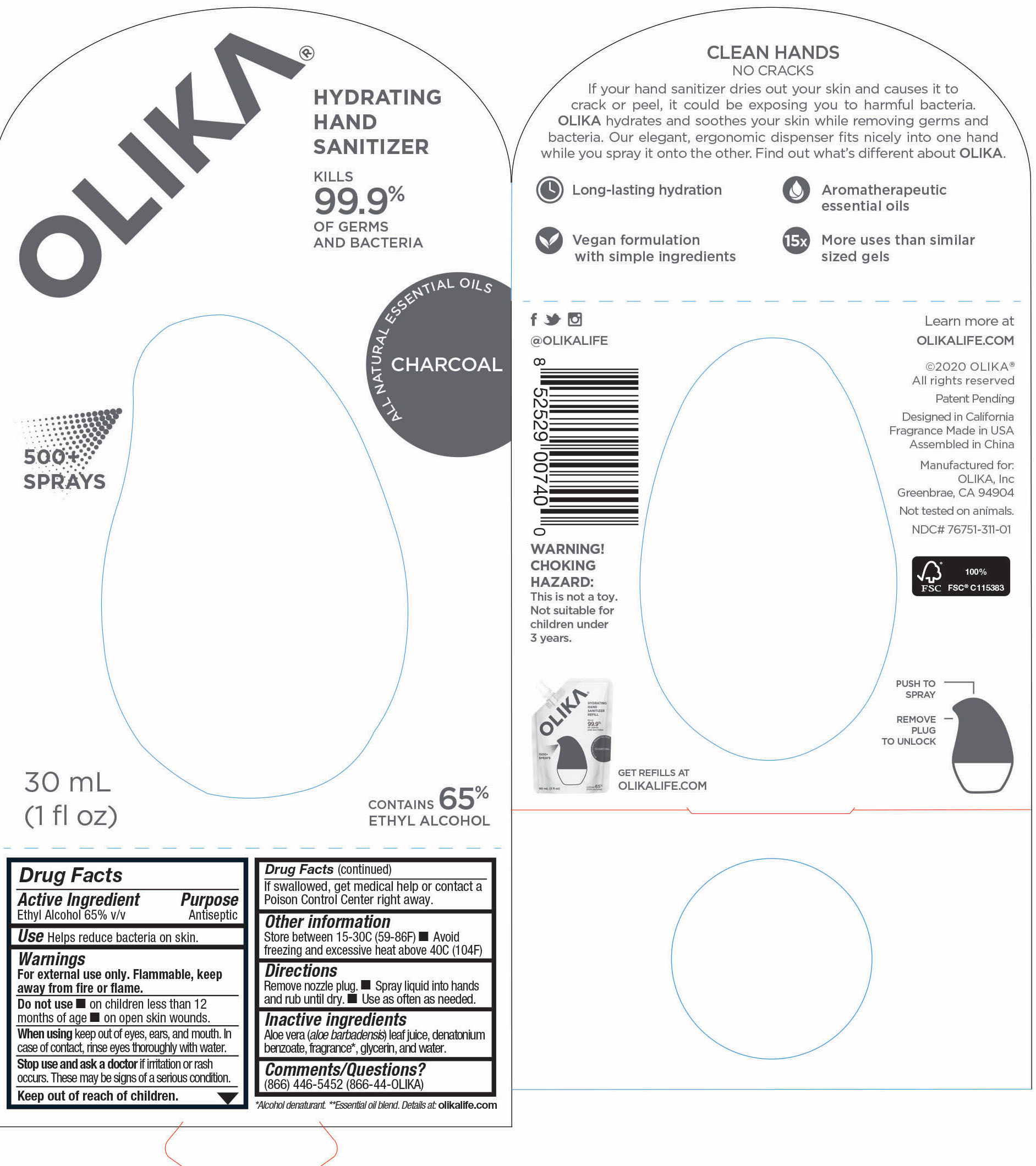
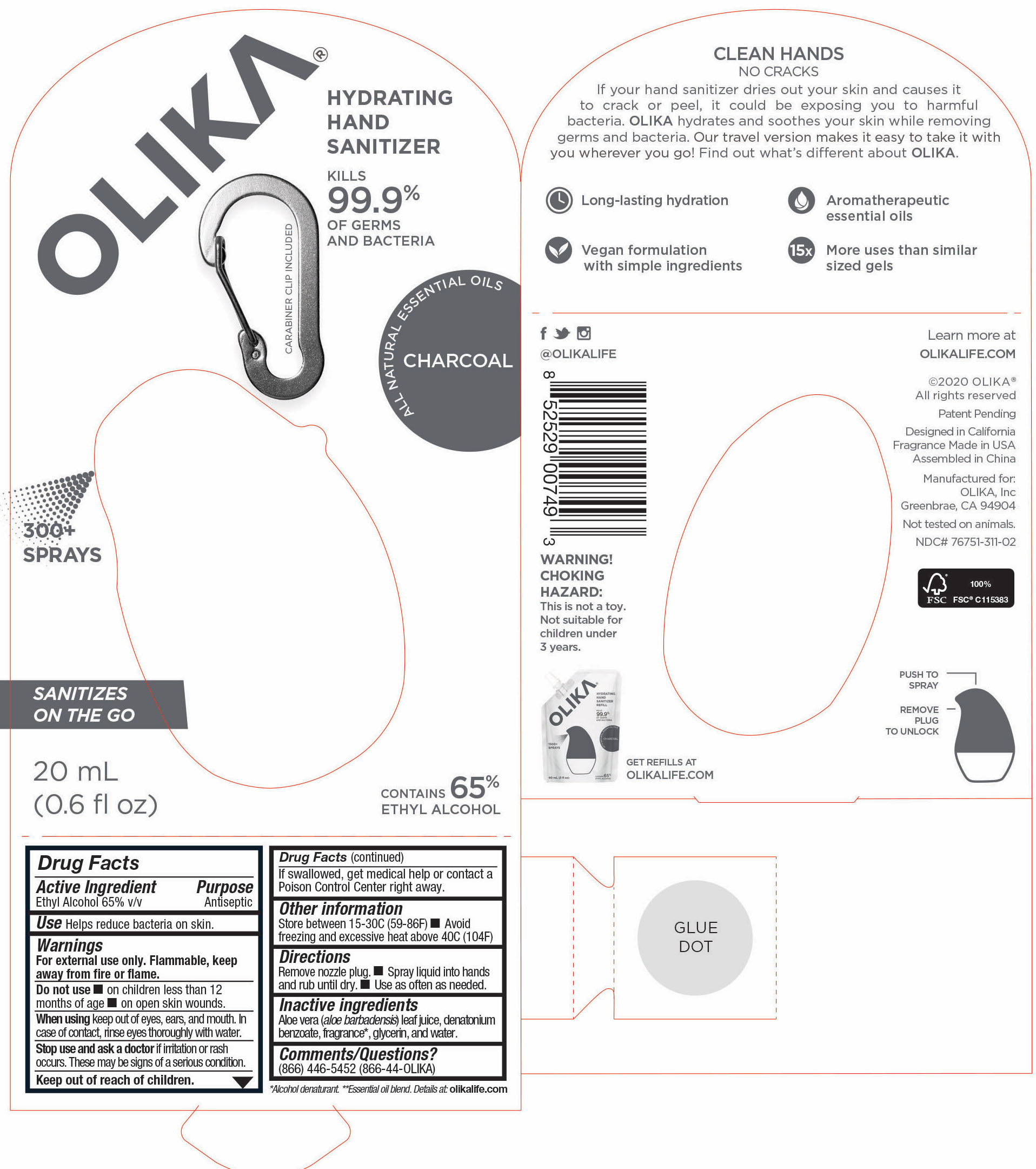
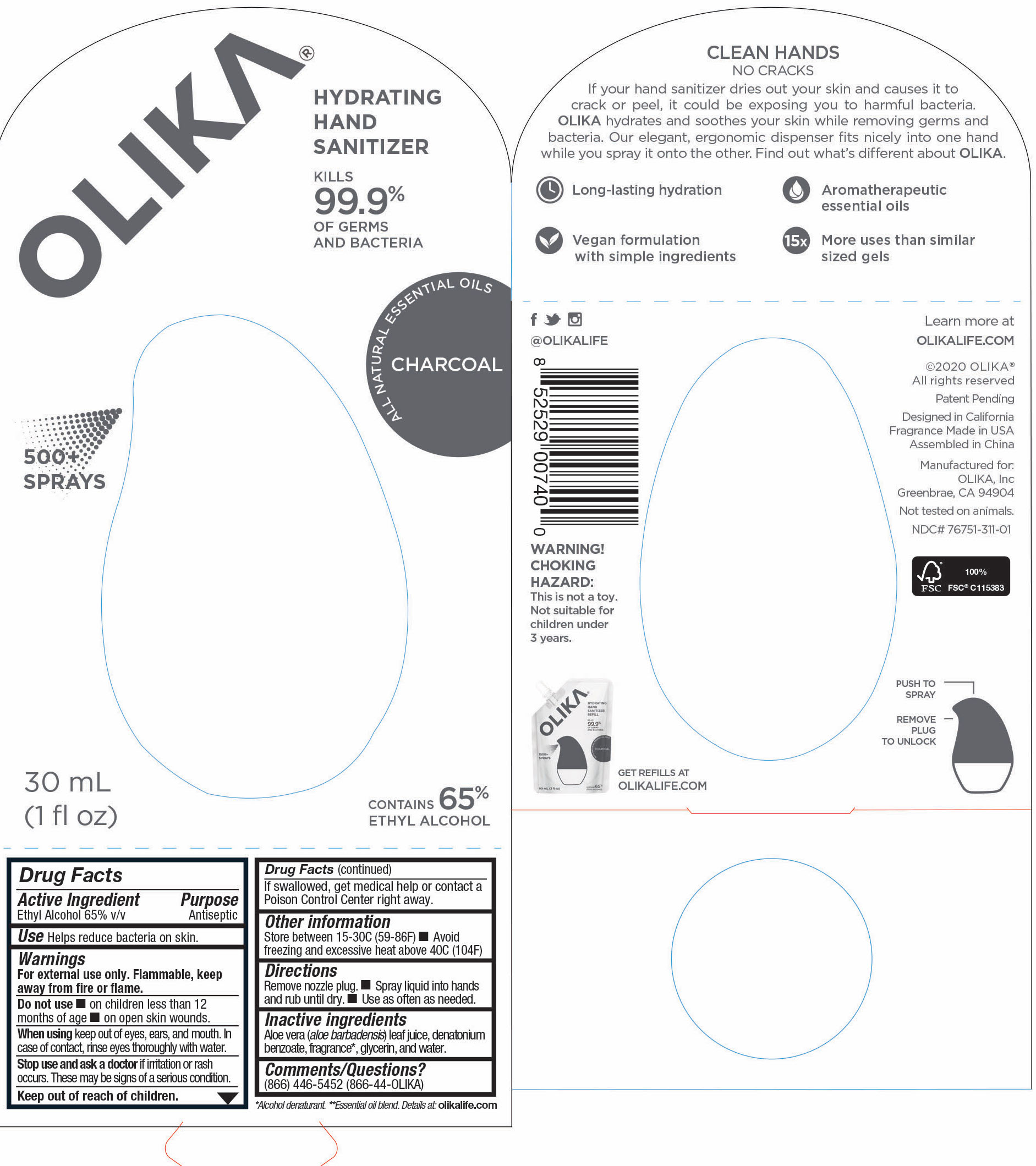
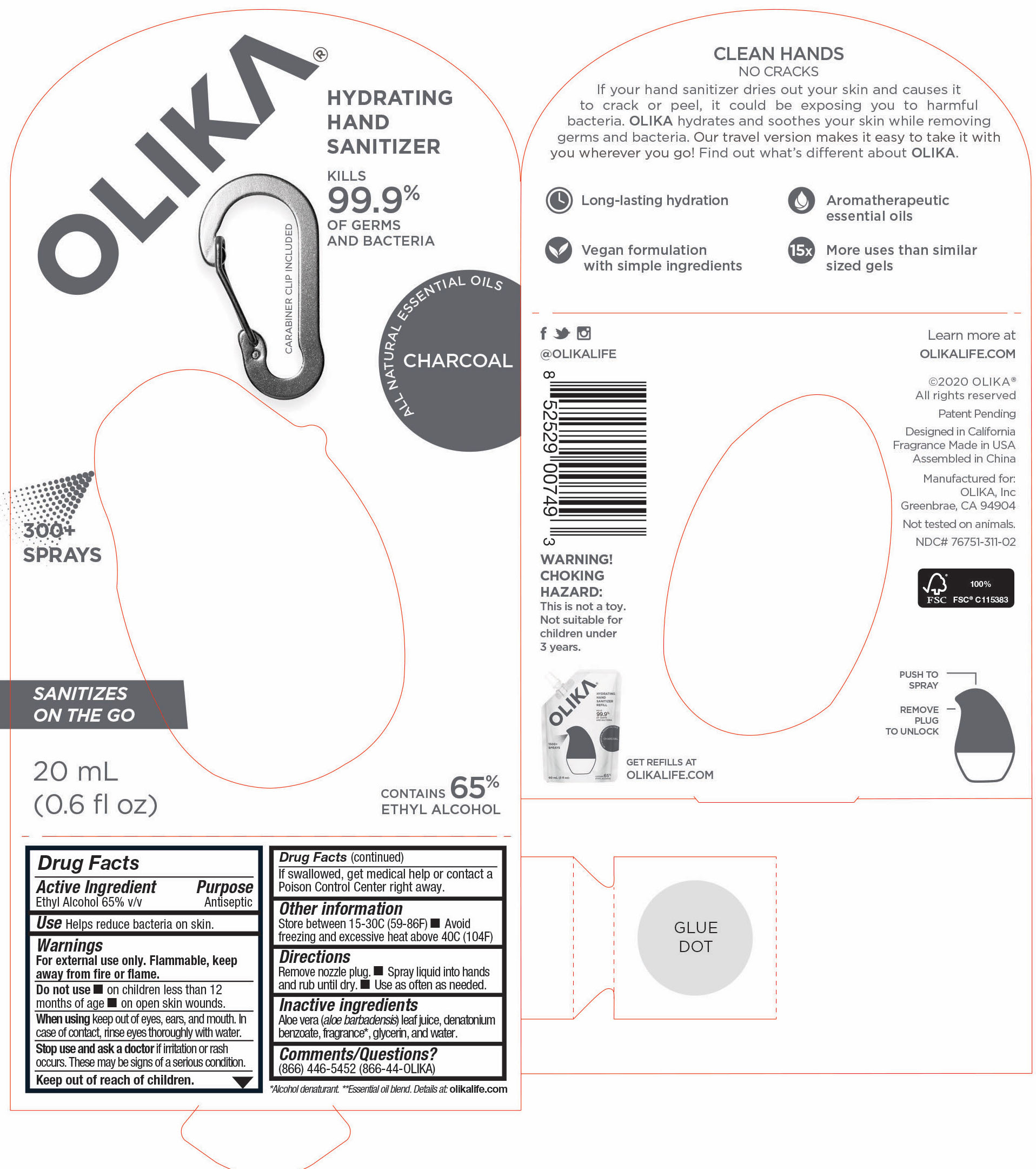
OLIKA HAND SANITIZER CHARCOAL- ethyl alcohol liquid
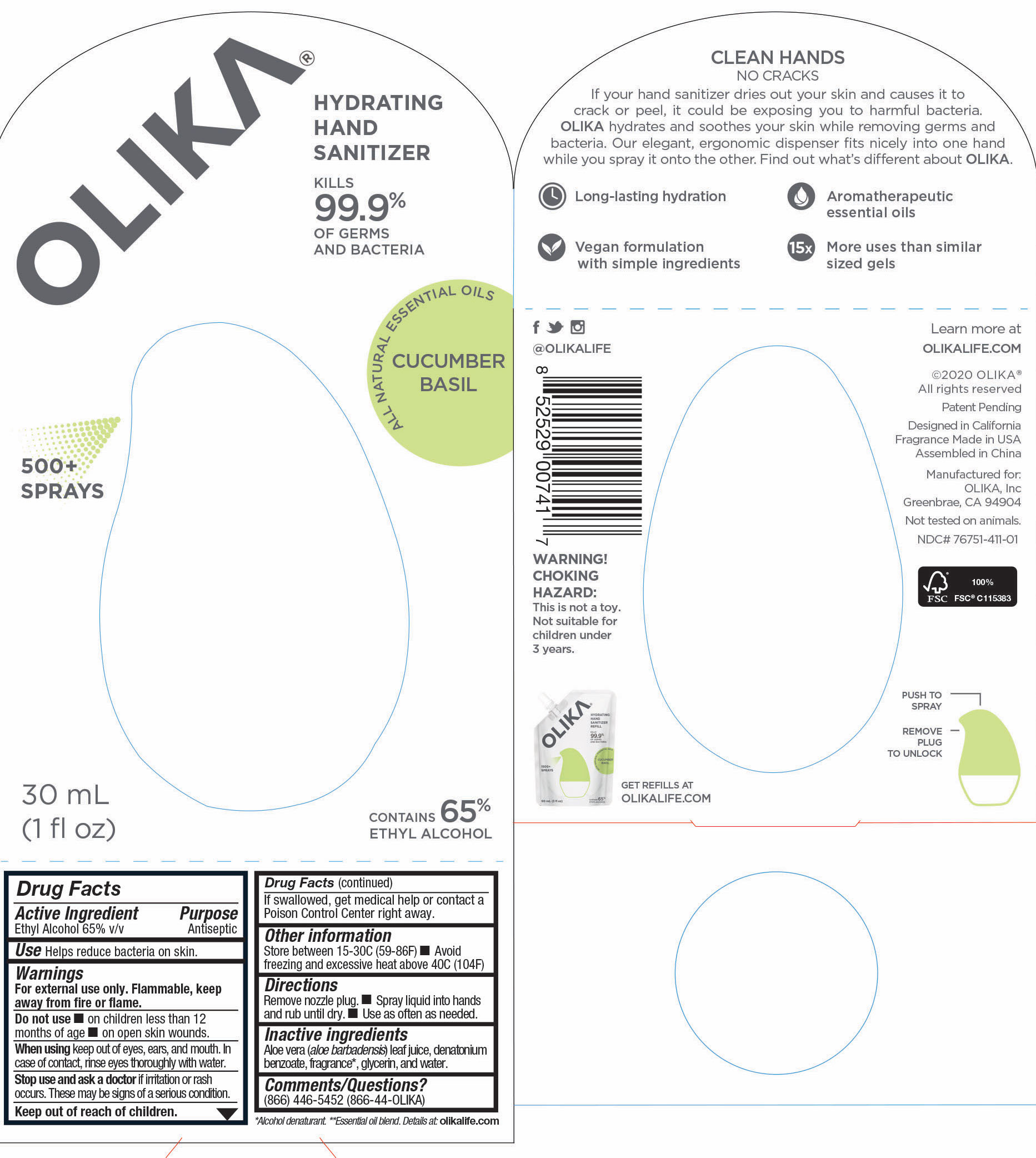
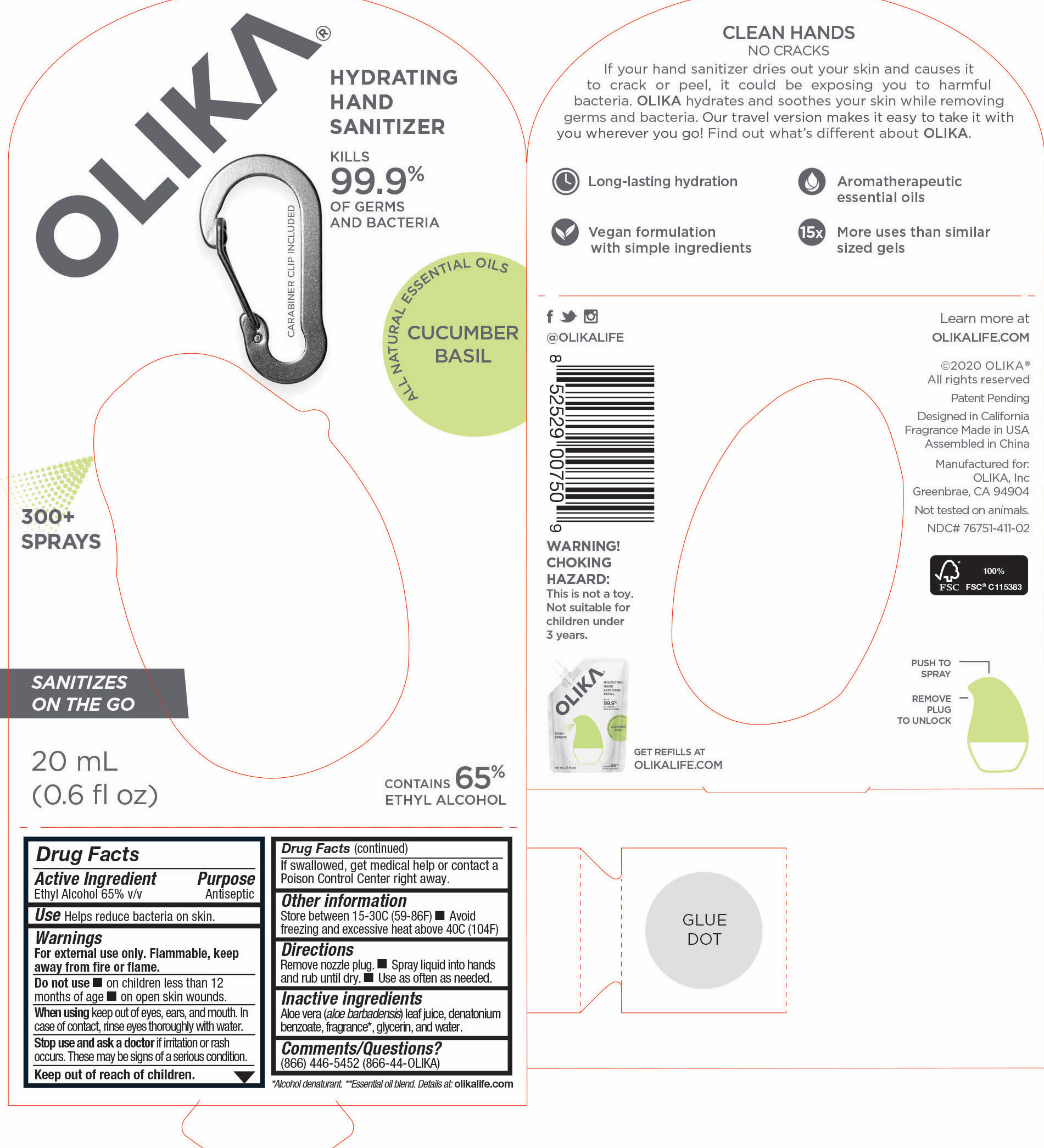
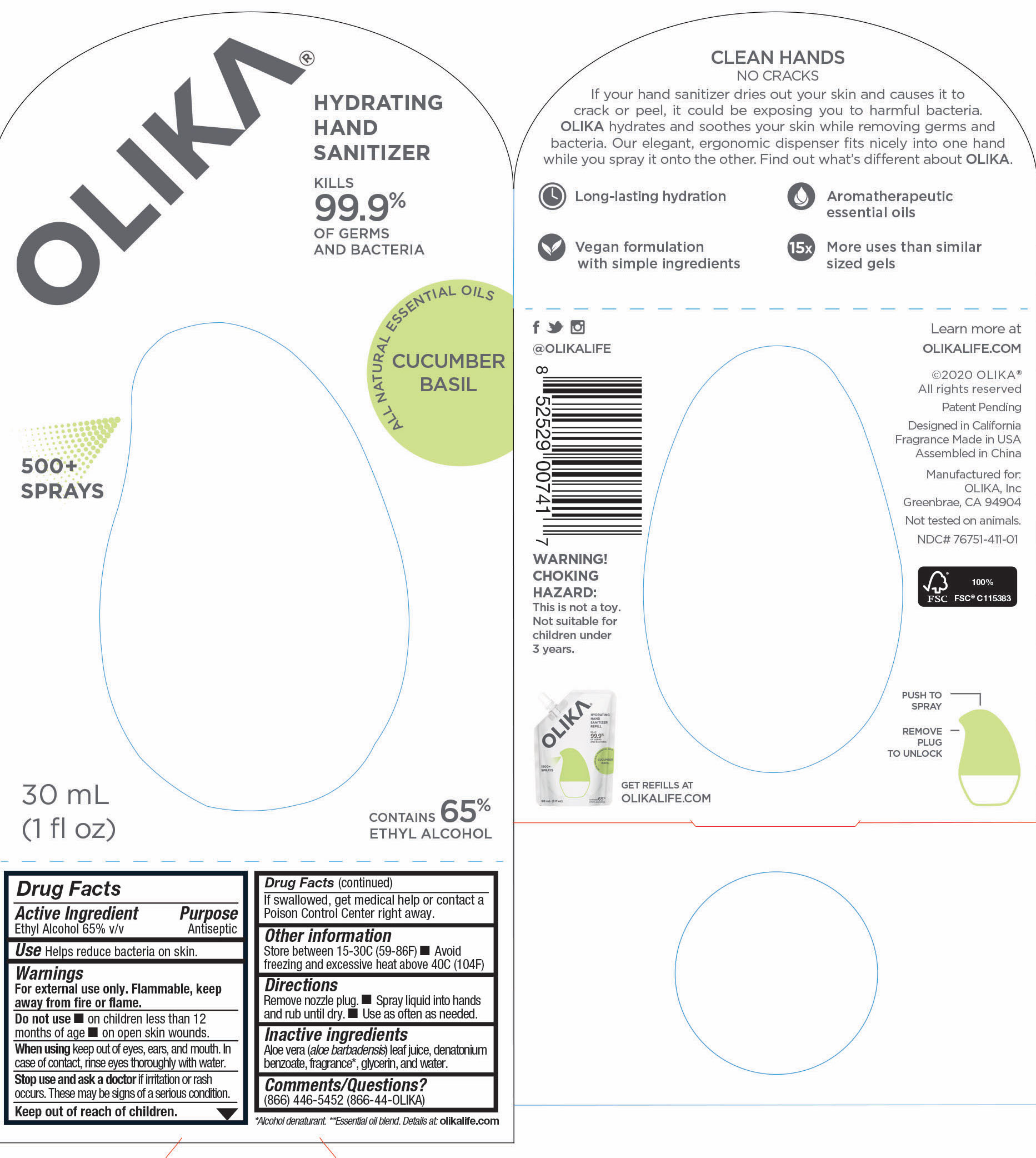
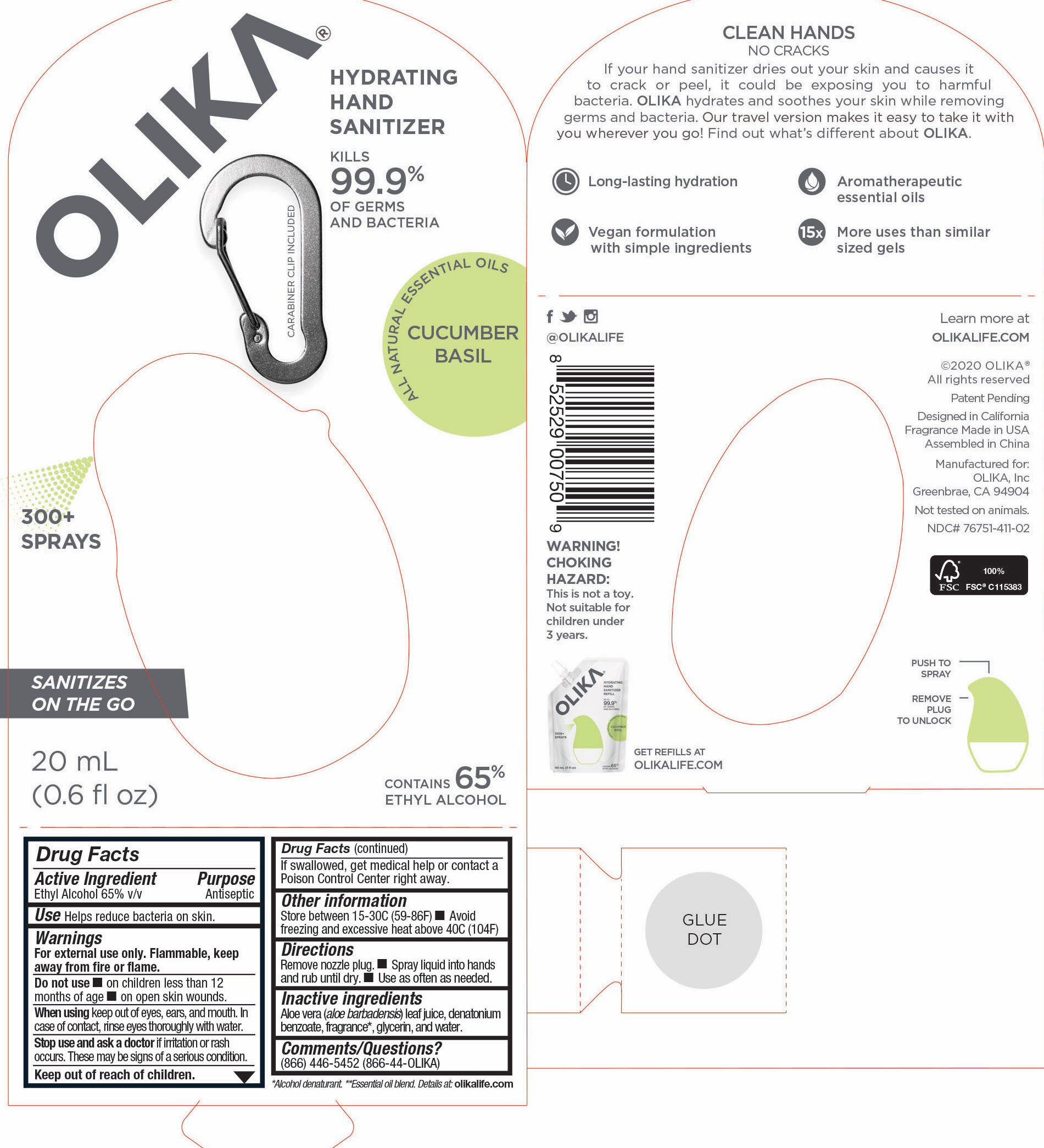
OLIKA HAND SANITIZER CUCUMBER BASIL- ethyl alcohol liquid
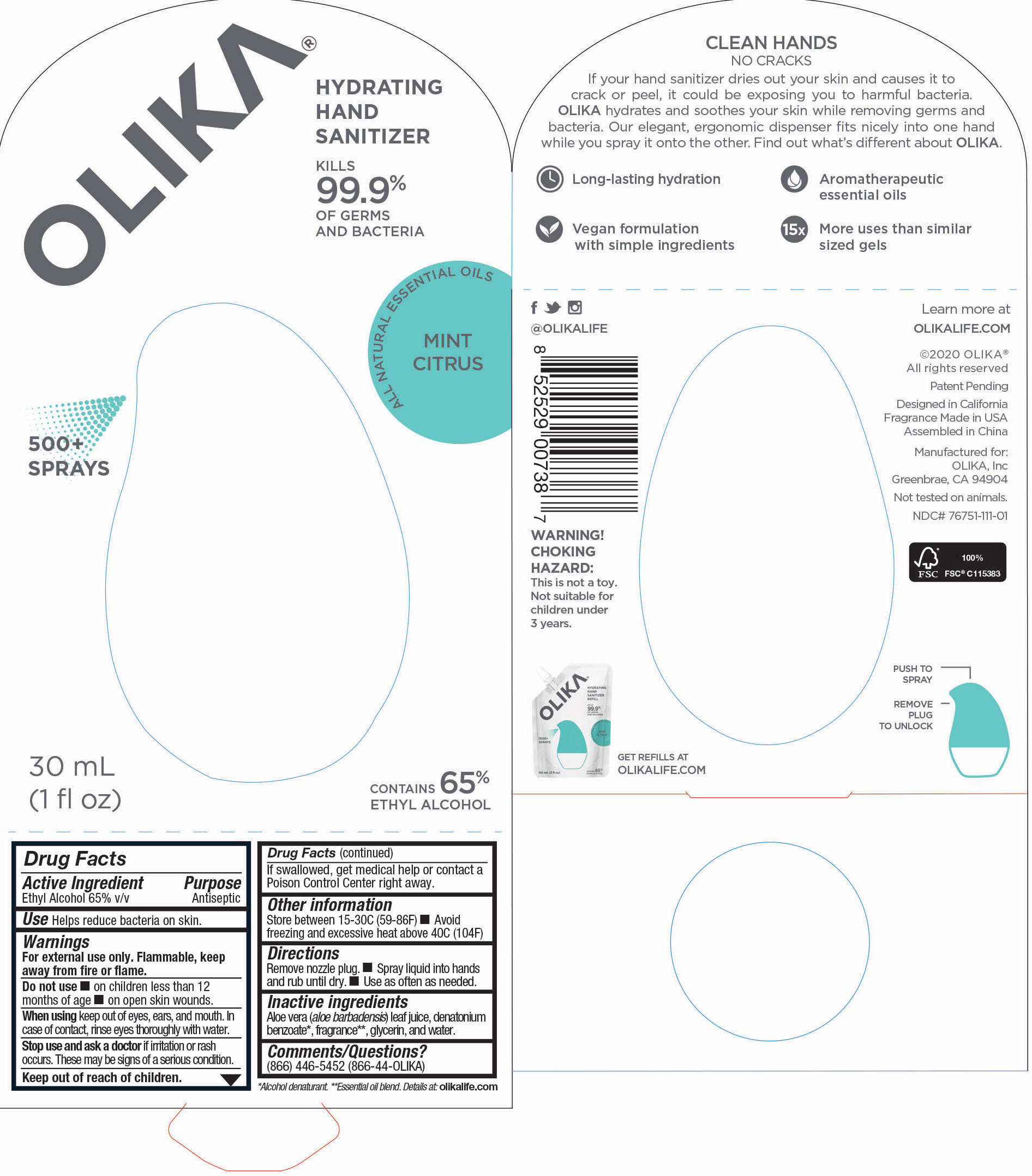
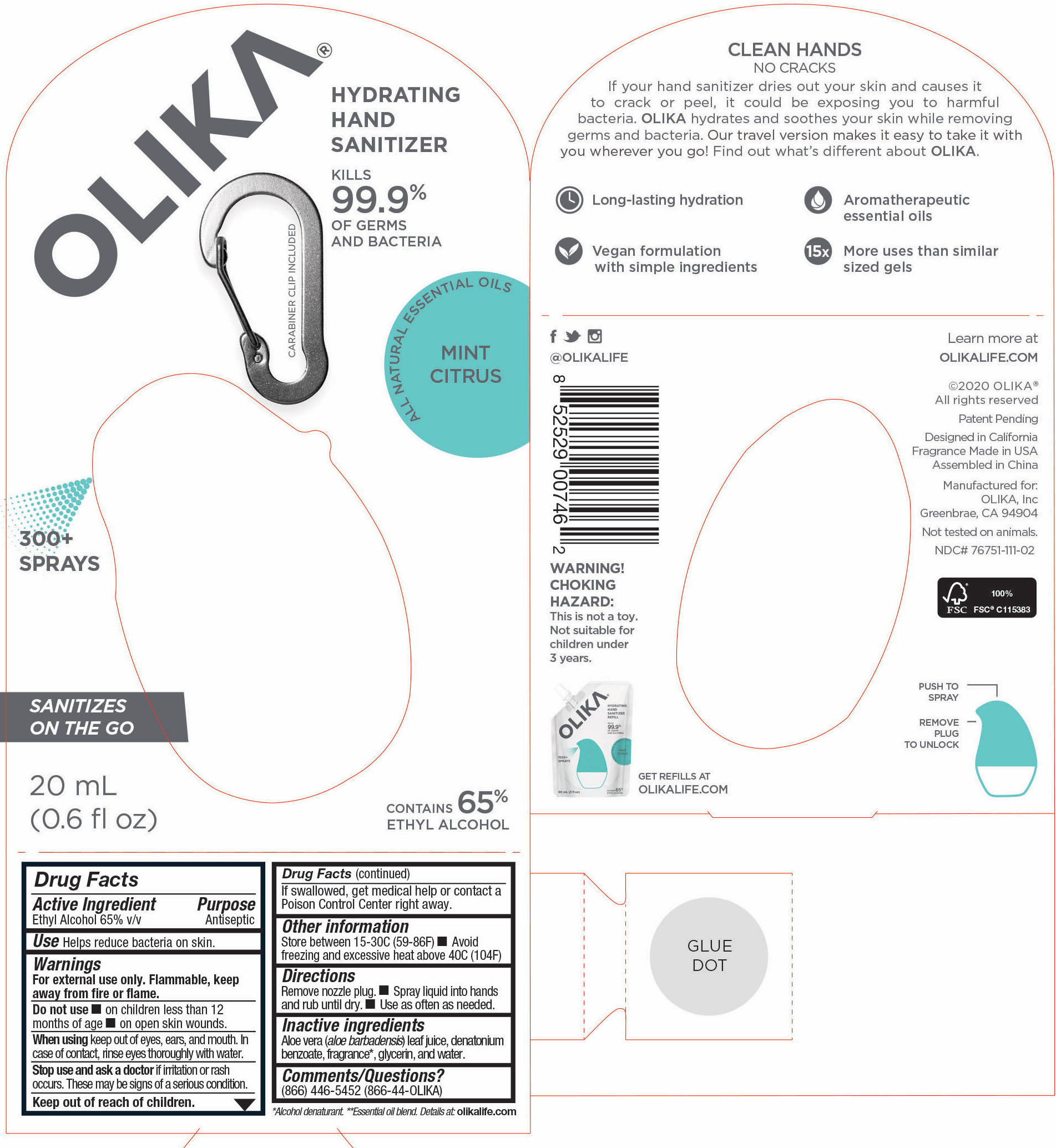
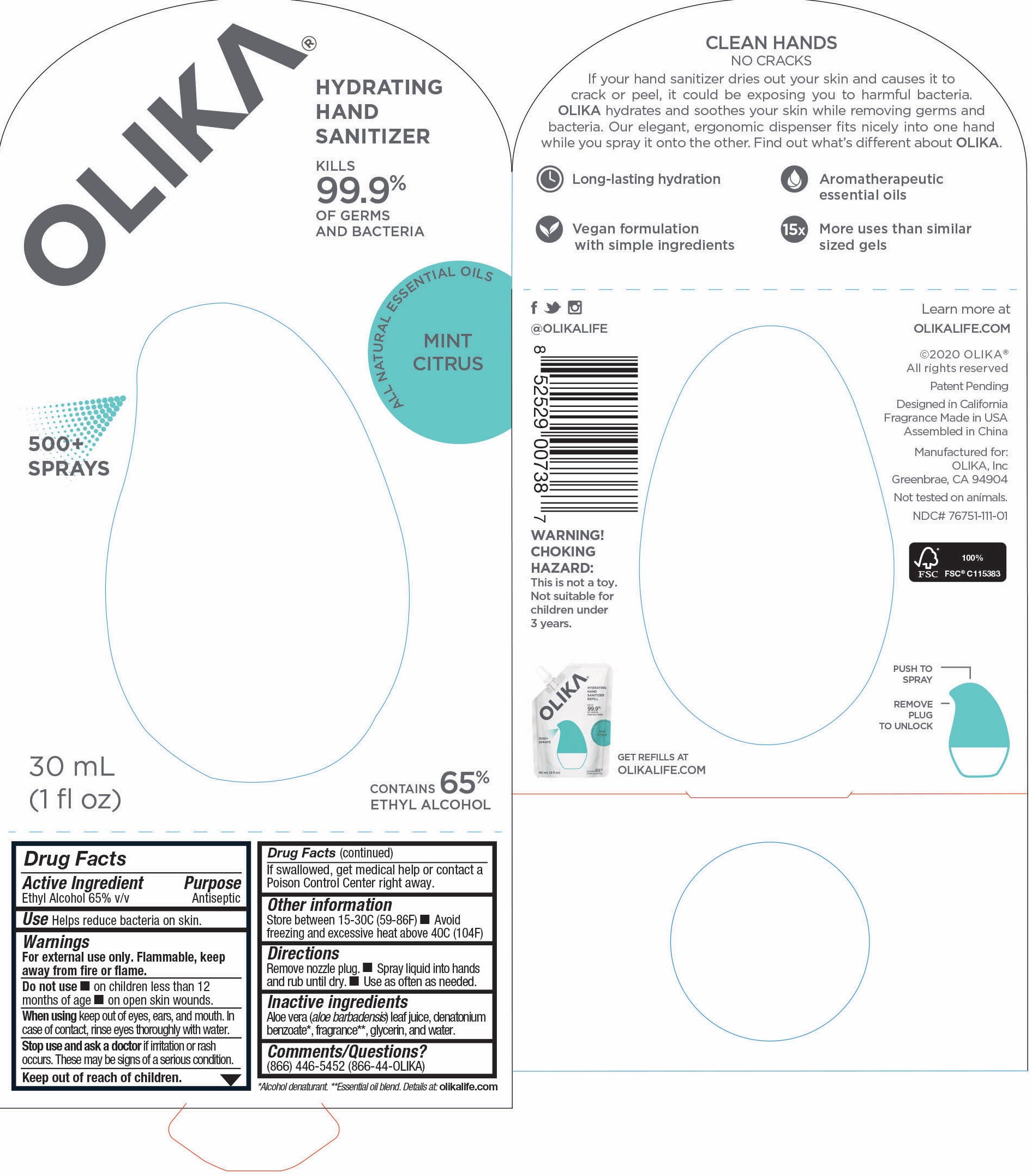
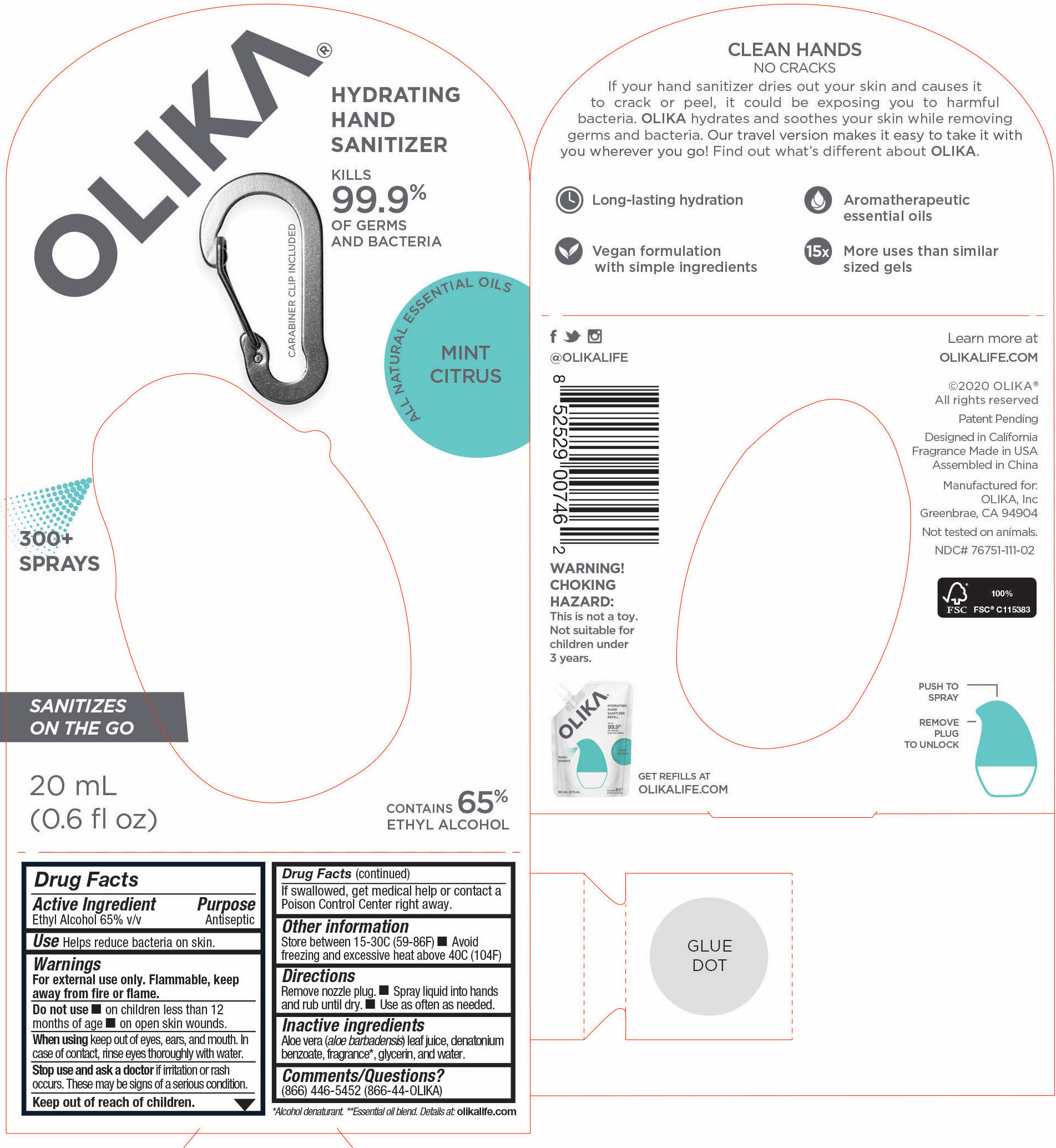
OLIKA HAND SANITIZER MINT CITRUS- ethyl alcohol liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 76751-111-01, 76751-111-02, 76751-111-03, 76751-311-01, view more76751-311-02, 76751-311-03, 76751-411-01, 76751-411-02, 76751-411-03, 76751-511-01, 76751-511-02, 76751-511-03, 76751-611-01, 76751-611-02, 76751-611-03 - Packager: Olika Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 10, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTION
These products are hand sanitizers manufactured according to the 1994 tentative final monograph for antiseptics with additional guidance taken from subsequent rule makings and guidances.
The hand sanitizer is manufactured using only the following United States Pharmacopoeia (USP) grade ingredients in the preparation of the product (percentage in final product formulation).
- Alcohol (ethanol) (USP or Food Chemical Codex (FCC) grade) (65%, volume/volume (v/v)) in an aqueous solution denatured according to Alcohol and Tobacco Tax and Trade Bureau regulations in 27 CFR part 20.
- Glycerol (4.2% v/v).
- Aloe Barbadensis Leaf Extract.
- Sterile distilled water or boiled cold water.
- Fragrance
The finished product formulation underwent third party efficacy testing (kill study) to confirm efficacy and product potency.
The hand sanitizer come in 6 different scents. Fragrance free (submitted separately NDC 76751-211), Lavender (NDC 76751-611), Orange Blossom (NDC 76751-511), Mint Citrus (NDC 76751-111), Charcoal (NDC 76751-311) and Cucumber Basil (NDC 76751-411).
- Active Ingredient(s)
- Purpose
- Warnings
- Do not use
-
WHEN USING
When using this product keep out of eyes, ears, and mouth. In case of contact with eyes, rinse eyes thoroughly with water.
Stop use and ask a doctor if irritation or rash occurs. These may be signs of a serious condition.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away. - STOP USE
- KEEP OUT OF REACH OF CHILDREN
- Other information
- Use
- Directions
- Inactive Ingredients
- Primary container spray bottle bottom label
- 76751-411-01 Prinicipal Display Panel and information panel image
- 76751-111-01 Prinicipal Display Panel and information Panel
- 76751-311-01 Image Principal Display Panel & Information Panel
- 76751-511-01 Principal Display panel and information panel
- 76751-111-02 Principal Display and Information Panel
- 76751-311-02 Principal Display and Information Panel
- 76751-611-02 PRINCIPAL DISPLAY PANEL AND INFORMATION LABEL
- 76751-411-02 PRINCIPAL DISPLAY PANEL AND INFORMATION PANEL
- 76751-111-03 PRINCIPAL DISCPLAY PANEL AND INFORMATION PANEL
- 76751-611-03 PRINCIPAL DISPLAY PANEL AND INFORMATION PANEL
- 76751-311-03 PRINCIPAL DISPLAY PANEL AND INFORMATION PANEL
- 76751-411-03 PRINCIPAL DISPLAY PANEL AND INFORMATION PANEL
- 76751-511-03 PRINCIPAL DISPLAY PANEL AND INFORMATON PANEL
- 76751-611-01 PRINCIPAL DISPLAY PANEL AND INFORMATION PANEL
- 76751-511-02 PRINCIPAL DISPLAY PANEL AND INFORMATION PANEL
-
INGREDIENTS AND APPEARANCE
OLIKA HAND SANITIZER ORANGE BLOSSOM
ethyl alcohol liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76751-511 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 65 mL in 100 mL Inactive Ingredients Ingredient Name Strength PIPERONAL (UNII: KE109YAK00) 0.0003 mL in 100 mL PELARGONIUM GRAVEOLENS FLOWER OIL (UNII: 3K0J1S7QGC) 0.0007 mL in 100 mL CITRUS AURANTIUM FRUIT OIL (UNII: 59JDQ5VT0T) 0.0005 mL in 100 mL TRIETHYL CITRATE (UNII: 8Z96QXD6UM) 0.3368 mL in 100 mL LINALOOL, (-)- (UNII: 3U21E3V8I2) 0.017 mL in 100 mL .ALPHA.-TERPINEOL (UNII: 21334LVV8W) 0.0048 mL in 100 mL PALMAROSA OIL (UNII: 0J3G3O53ST) 0.0096 mL in 100 mL GLYCERIN (UNII: PDC6A3C0OX) 4.2 mL in 100 mL ALOE VERA LEAF (UNII: ZY81Z83H0X) 0.25 mL in 100 mL WATER (UNII: 059QF0KO0R) 30.05 mL in 100 mL MALTOL (UNII: 3A9RD92BS4) 0.0003 mL in 100 mL .DELTA.-DODECALACTONE (UNII: 33DIC582TL) 0.0002 mL in 100 mL BENZYL SALICYLATE (UNII: WAO5MNK9TU) 0.0013 mL in 100 mL LITSEA OIL (UNII: 2XIW34BN6O) 0.0032 mL in 100 mL ORANGE OIL (UNII: AKN3KSD11B) 0.0028 mL in 100 mL 2,6-DIMETHYL-5-HEPTENAL (UNII: Z331YX9EL9) 0.0002 mL in 100 mL .DELTA.-DECALACTONE (UNII: CNA0S5T234) 0.0013 mL in 100 mL PHENYLETHYL ALCOHOL (UNII: ML9LGA7468) 0.0006 mL in 100 mL VANILLIN (UNII: CHI530446X) 0.0005 mL in 100 mL CARDAMOM OIL (UNII: JM0KJ091HZ) 0.0002 mL in 100 mL METHYL ANTHRANILATE (UNII: 981I0C1E5W) 0.001 mL in 100 mL CITRUS SINENSIS LEAF (UNII: VF01D90MZI) 0.0084 mL in 100 mL CANANGA ODORATA FLOWER (UNII: 76GTF6Z97M) 0.0029 mL in 100 mL .GAMMA.-OCTALACTONE (UNII: UHD6M52X0K) 0.0005 mL in 100 mL BERGAMOT OIL (UNII: 39W1PKE3JI) 0.0112 mL in 100 mL POGOSTEMON CABLIN LEAF OIL (UNII: F3IN55X5PO) 0.0006 mL in 100 mL .GAMMA.-DECALACTONE (UNII: 7HLS05KP9O) 0.0006 mL in 100 mL MANDARIN OIL (UNII: NJO720F72R) 0.0016 mL in 100 mL CLOVE LEAF OIL (UNII: VCA5491KVF) 0.0026 mL in 100 mL CITRUS SINENSIS FLOWER OIL (UNII: AJ56JP5TFP) 0.0888 mL in 100 mL ALLYL HEXANOATE (UNII: 3VH84A363D) 0.0013 mL in 100 mL LEMON OIL (UNII: I9GRO824LL) 0.0002 mL in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76751-511-03 90 mL in 1 POUCH; Type 0: Not a Combination Product 06/01/2020 2 NDC:76751-511-01 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 06/01/2020 3 NDC:76751-511-02 20 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 06/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 06/01/2020 OLIKA HAND SANITIZER LAVENDER
ethyl alcohol liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76751-611 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 65 mL in 100 mL Inactive Ingredients Ingredient Name Strength SPEARMINT OIL (UNII: C3M81465G5) 0.005991 mL in 100 mL GLYCERIN (UNII: PDC6A3C0OX) 4.2 mL in 100 mL ALOE VERA LEAF (UNII: ZY81Z83H0X) 0.25 mL in 100 mL WATER (UNII: 059QF0KO0R) 30.05 mL in 100 mL DIPTERYX ODORATA SEED (UNII: D43A5L1U6L) 0.013825 mL in 100 mL VANILLIN (UNII: CHI530446X) 0.0013825 mL in 100 mL TRIETHYL CITRATE (UNII: 8Z96QXD6UM) 0.3364055 mL in 100 mL LAVANDIN OIL (UNII: 9RES347CKG) 0.0557605 mL in 100 mL LEMON OIL (UNII: I9GRO824LL) 0.0073735 mL in 100 mL LINALOOL, (-)- (UNII: 3U21E3V8I2) 0.00599 mL in 100 mL AMYRIS BALSAMIFERA OIL (UNII: I1BJ961J2E) 0.002765 mL in 100 mL POGOSTEMON CABLIN LEAF OIL (UNII: F3IN55X5PO) 0.010599 mL in 100 mL BULNESIA SARMIENTOI WOOD OIL (UNII: 81H0L6W02F) 0.032258 mL in 100 mL EUCALYPTUS OIL (UNII: 2R04ONI662) 0.0152075 mL in 100 mL JUNIPERUS DEPPEANA WOOD OIL (UNII: 4739QA5686) 0.0124425 mL in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76751-611-03 90 mL in 1 POUCH; Type 0: Not a Combination Product 06/01/2020 2 NDC:76751-611-01 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 06/01/2020 3 NDC:76751-611-02 20 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 06/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 06/01/2020 OLIKA HAND SANITIZER CHARCOAL
ethyl alcohol liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76751-311 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 65.1846 mL in 100 mL Inactive Ingredients Ingredient Name Strength BENZOIN RESIN (UNII: GK21SBA74R) 0.0013 mL in 100 mL LINALYL ACETATE (UNII: 5K47SSQ51G) 0.0074 mL in 100 mL JUNIPERUS VIRGINIANA OIL (UNII: PAD4FN7P2G) 0.0176 mL in 100 mL GLYCERIN (UNII: PDC6A3C0OX) 4.2 mL in 100 mL ALOE VERA LEAF (UNII: ZY81Z83H0X) 0.25 mL in 100 mL WATER (UNII: 059QF0KO0R) 30.05 mL in 100 mL LINALOOL, (-)- (UNII: 3U21E3V8I2) 0.0098 mL in 100 mL VANILLIN (UNII: CHI530446X) 0.0028 mL in 100 mL LEMON OIL (UNII: I9GRO824LL) 0.0113 mL in 100 mL CLARY SAGE OIL (UNII: 87L0D4U3M0) 0.0007 mL in 100 mL BERGAMOT OIL (UNII: 39W1PKE3JI) 0.0078 mL in 100 mL VETIVER OIL (UNII: 9M9P32M01L) 0.0002 mL in 100 mL TRIETHYL CITRATE (UNII: 8Z96QXD6UM) 0.242 mL in 100 mL POGOSTEMON CABLIN LEAF OIL (UNII: F3IN55X5PO) 0.0061 mL in 100 mL AMYRIS BALSAMIFERA OIL (UNII: I1BJ961J2E) 0.003 mL in 100 mL BENZYL ACETATE (UNII: 0ECG3V79ZJ) 0.0026 mL in 100 mL LABDANUM OIL (UNII: 67GS9BGA2X) 0.0019 mL in 100 mL .BETA.-IONONE (UNII: A7NRR1HLH6) 0.0009 mL in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76751-311-03 90 mL in 1 POUCH; Type 0: Not a Combination Product 06/01/2020 2 NDC:76751-311-01 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 06/01/2020 3 NDC:76751-311-02 20 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 06/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 06/01/2020 OLIKA HAND SANITIZER CUCUMBER BASIL
ethyl alcohol liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76751-411 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 65.2459 mL in 100 mL Inactive Ingredients Ingredient Name Strength CUCUMBER JUICE (UNII: 61845009SP) 0.0074 mL in 100 mL SWEET VIOLET LEAF OIL (UNII: 227KB7O2KN) 0.0002 mL in 100 mL BASIL OIL (UNII: Z129UMU8LE) 0.0007 mL in 100 mL JUNIPERUS VIRGINIANA OIL (UNII: PAD4FN7P2G) 0.0032 mL in 100 mL MIMOSA TENUIFLORA BARK (UNII: 515MQE449I) 0.0001 mL in 100 mL 3-HEXEN-1-OL, (3Z)- (UNII: V14F8G75P4) 0.0012 mL in 100 mL EUGENOL (UNII: 3T8H1794QW) 0.0007 mL in 100 mL .GAMMA.-DECALACTONE (UNII: 7HLS05KP9O) 0.0007 mL in 100 mL LIMONENE, (+)- (UNII: GFD7C86Q1W) 0.0176 mL in 100 mL LINALOOL, (-)- (UNII: 3U21E3V8I2) 0.0147 mL in 100 mL CITRUS SINENSIS FLOWER OIL (UNII: AJ56JP5TFP) 0.0206 mL in 100 mL HEXYL ACETATE (UNII: 7U7KU3MWT0) 0.0018 mL in 100 mL 2,6-DIMETHYL-5-HEPTENAL (UNII: Z331YX9EL9) 0.0012 mL in 100 mL CITRUS AURANTIUM FRUIT OIL (UNII: 59JDQ5VT0T) 0.0009 mL in 100 mL TRIETHYL CITRATE (UNII: 8Z96QXD6UM) 0.1779 mL in 100 mL LITSEA OIL (UNII: 2XIW34BN6O) 0.0029 mL in 100 mL ORANGE OIL (UNII: AKN3KSD11B) 0.0001 mL in 100 mL NEROLIDOL (UNII: QR6IP857S6) 0.0006 mL in 100 mL GLYCERIN (UNII: PDC6A3C0OX) 4.2 mL in 100 mL ALOE VERA LEAF (UNII: ZY81Z83H0X) 0.25 mL in 100 mL WATER (UNII: 059QF0KO0R) 30.05 mL in 100 mL ANETHOLE (UNII: Q3JEK5DO4K) 0.0015 mL in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76751-411-03 90 mL in 1 POUCH; Type 0: Not a Combination Product 06/01/2020 2 NDC:76751-411-01 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 06/01/2020 3 NDC:76751-411-02 20 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 06/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 06/01/2020 OLIKA HAND SANITIZER MINT CITRUS
ethyl alcohol liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76751-111 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 65 mL in 100 mL Inactive Ingredients Ingredient Name Strength LEMON OIL (UNII: I9GRO824LL) 0.01923 mL in 100 mL CARAWAY OIL (UNII: C2J9B08Q3I) 0.0769 mL in 100 mL GRAPEFRUIT OIL (UNII: YR377U58W9) 0.0077 mL in 100 mL TRIETHYL CITRATE (UNII: 8Z96QXD6UM) 0.5577 mL in 100 mL BERGAMOT OIL (UNII: 39W1PKE3JI) 0.1462 mL in 100 mL SPEARMINT OIL (UNII: C3M81465G5) 0.0192 mL in 100 mL GLYCERIN (UNII: PDC6A3C0OX) 4.2 mL in 100 mL ALOE VERA LEAF (UNII: ZY81Z83H0X) 0.25 mL in 100 mL WATER (UNII: 059QF0KO0R) 29.55 mL in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76751-111-03 90 mL in 1 POUCH; Type 0: Not a Combination Product 06/01/2020 2 NDC:76751-111-01 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 06/01/2020 3 NDC:76751-111-02 20 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 06/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 06/01/2020 Labeler - Olika Inc. (080476192)