Label: FLU EASE- cairina moschata heart/liver autolysate pellet
- NDC Code(s): 42681-9280-3, 42681-9280-4, 42681-9281-1
- Packager: WFM Private Label, LP
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated October 4, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
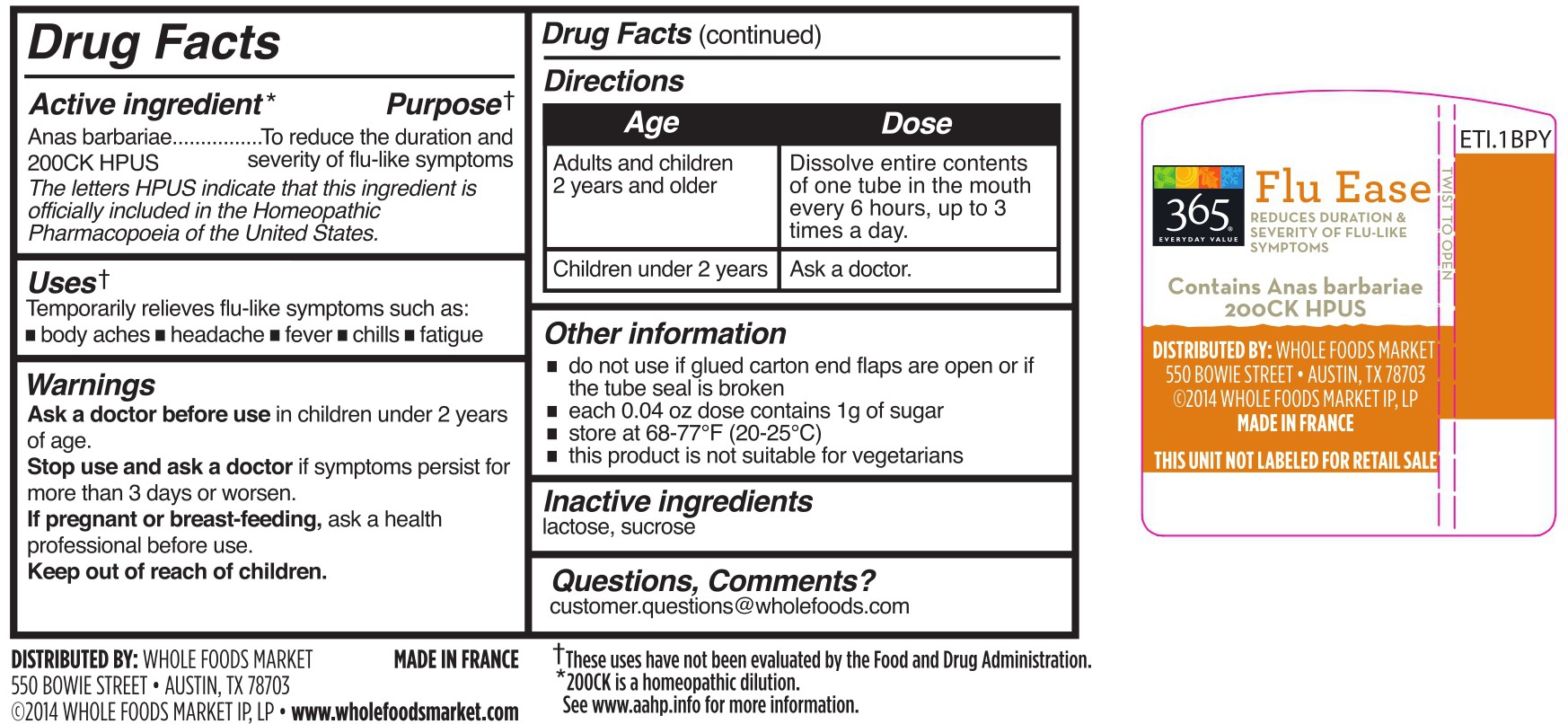
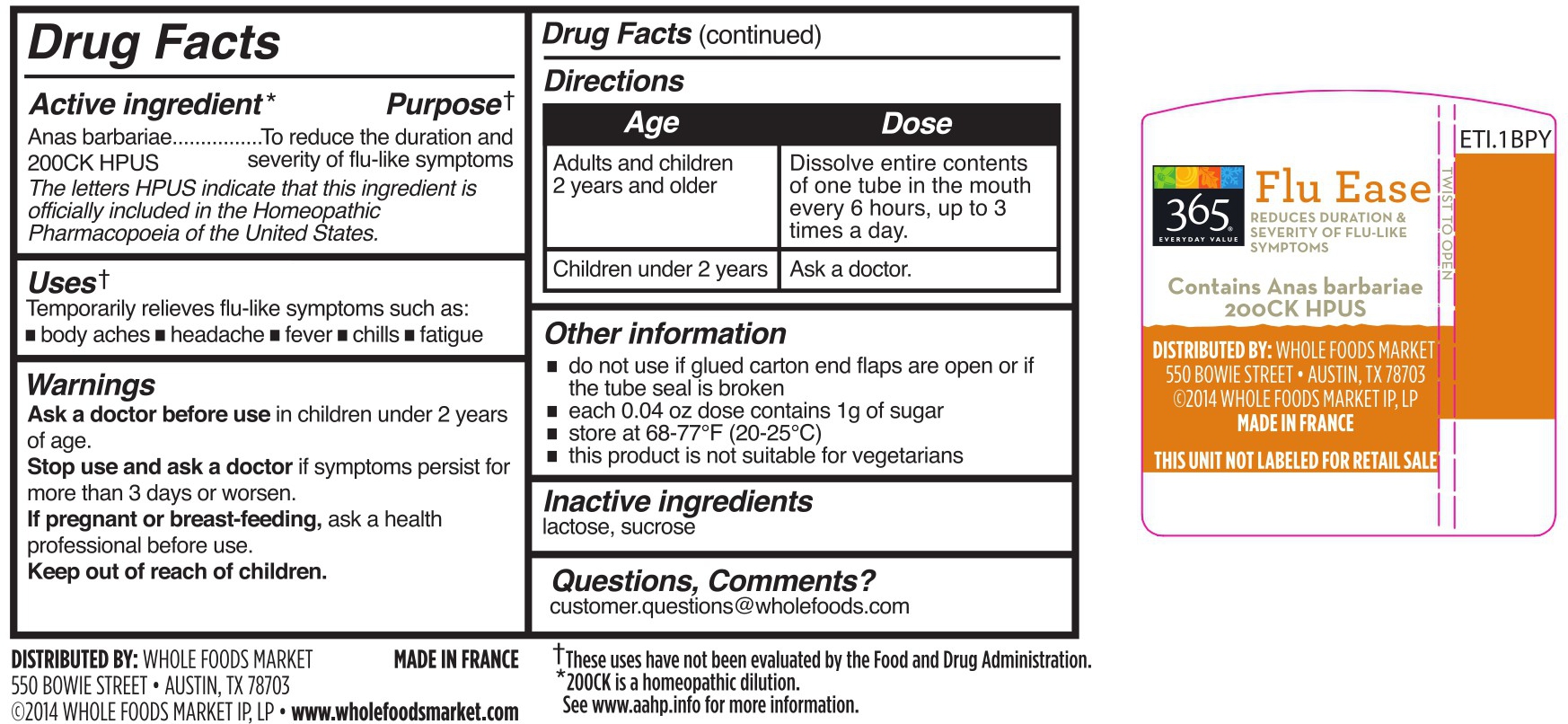
- DRUG FACTS
- Active Ingredient
- Purpose
- Uses
- WARNINGS
- Directions
-
Other information
do not use if glued carton end flaps are open or if the tube seal is broken
each 0.04 oz does contains 1g of sugar
this product is not suitable for vegetarians
Relieves Body Aches, Headache, Fever & Chills*
No Known Drug Interactions
Non-Drowsy
18 Doses
6 Doses
Dissolvable Oral Pellets
*THESE "USES" HAVE NOT BEEN EVALUATED BY THE FOOD AND DRUG ADMINISTRATION.
**200CK is a homeopathic dilution. See www.THEAAHP.org for more informaiton.
Distributed by: Whole Foods Market
550 Bowie Street, Austin, TX 78703
© 2020 WHOLE FOODS MARKET IP, LP
www.wholefoodsmarket.com
Made in France
- Inactive ingredients
- Questions, Comments?
- Principle Display Panel
-
INGREDIENTS AND APPEARANCE
FLU EASE
cairina moschata heart/liver autolysate pelletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:42681-9280 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAIRINA MOSCHATA HEART/LIVER AUTOLYSATE (UNII: RN2HC612GY) (CAIRINA MOSCHATA HEART/LIVER AUTOLYSATE - UNII:RN2HC612GY) CAIRINA MOSCHATA HEART/LIVER AUTOLYSATE 200 [hp_C] Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) LACTOSE (UNII: J2B2A4N98G) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42681-9280-4 6 in 1 CARTON 01/01/2015 1 200 in 1 TUBE; Type 0: Not a Combination Product 2 NDC:42681-9280-3 200 in 1 TUBE; Type 0: Not a Combination Product 01/01/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/01/2015 FLU EASE
cairina moschata heart/liver autolysate pelletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:42681-9281 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAIRINA MOSCHATA HEART/LIVER AUTOLYSATE (UNII: RN2HC612GY) (CAIRINA MOSCHATA HEART/LIVER AUTOLYSATE - UNII:RN2HC612GY) CAIRINA MOSCHATA HEART/LIVER AUTOLYSATE 200 [hp_C] Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) LACTOSE (UNII: J2B2A4N98G) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42681-9281-1 18 in 1 CARTON 01/01/2015 1 200 in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/01/2015 Labeler - WFM Private Label, LP (196175616)




 Drug Facts / Tube
Drug Facts / Tube