Label: HAND SANITIZER- ethyl alcohol gel
-
NDC Code(s):
75360-1125-1,
75360-1125-2,
75360-1125-3,
75360-1125-4, view more75360-1125-5, 75360-1125-6, 75360-1125-7
- Packager: Hemani International
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 19, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTION
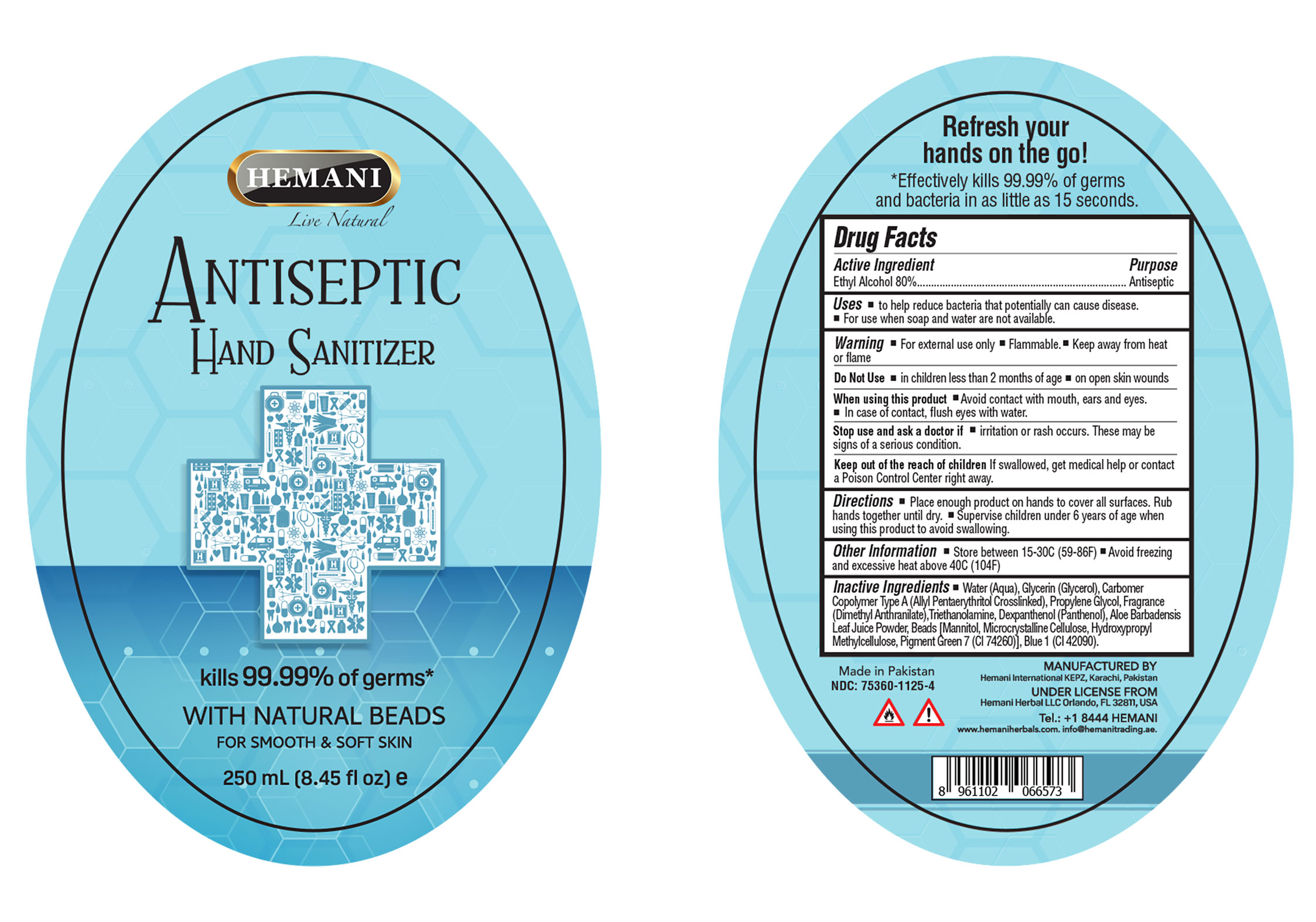
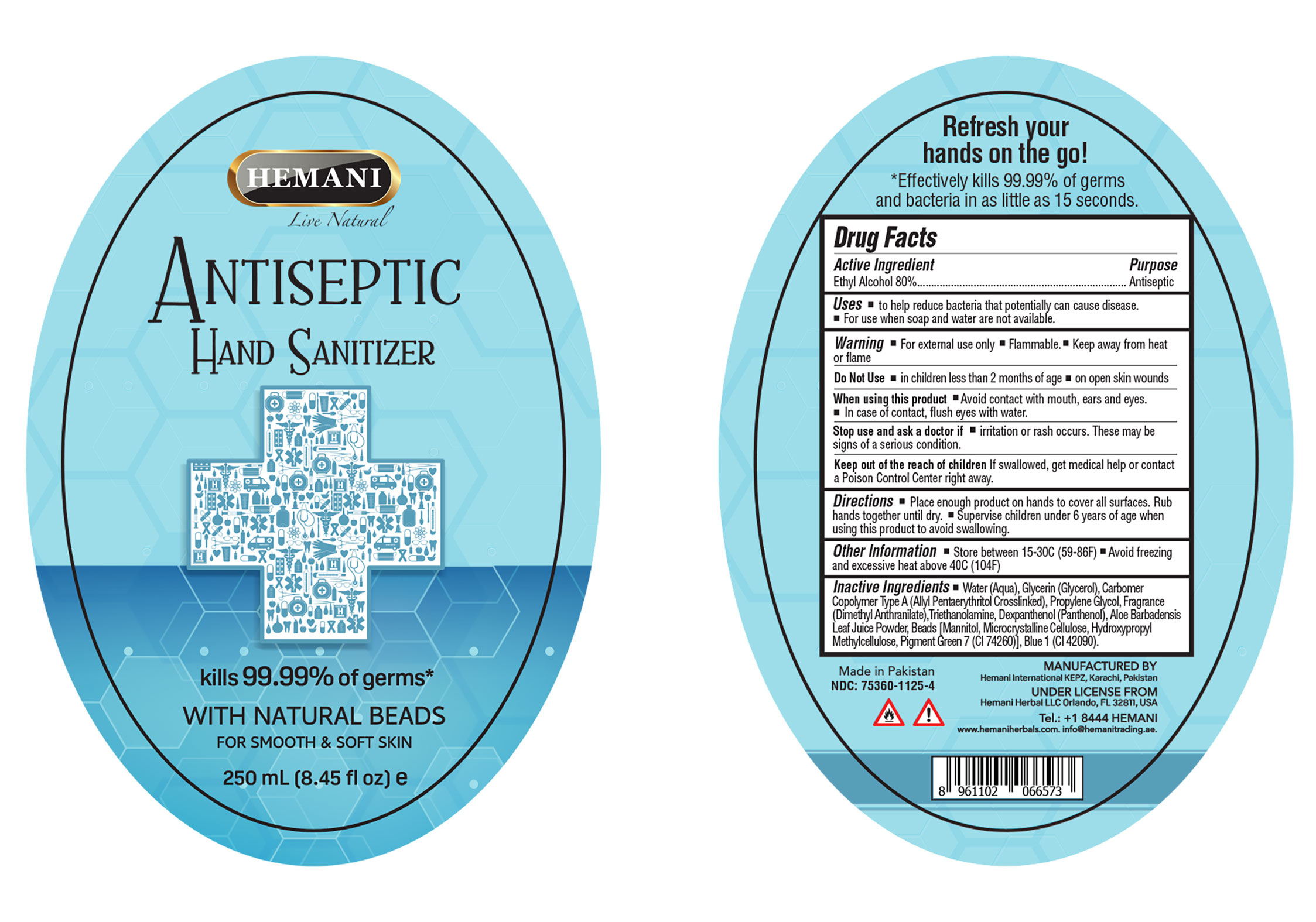
The hand sanitizer is manufactured using only the following ingredients in the preparation of the product (percentage in final product formulation):
- Alcohol (ethanol) (USP or Food Chemical Codex (FCC) grade) (80%, volume/volume (v/v))
- Sterile distilled water or boiled cold water.
- Glycerol (5% v/v).
- Carbomer Copolymer Type A (Allyl Pentaerythritol Crosslinked)(1.5% v/v).
- Propylene Glycol (1% v/v).
- Fragrance (Dimethyl Anthranilate) (0.5% v/v).
- Triethanolamine (0.15% v/v).
- Dexpanthenol (Panthenol) (0.10% v/v).
- Aloe Barbadensis Leaf Juice Powder (0.05% v/v).
- Beads (Mannitol, Microcrystalline Cellulose, Hydroxypropyl Methylcellulose, Pigment Green 7 (CI 74260) (0.02% v/v).
- Blue 1 (CI 42090) (0.005% v/v).
The firm does not add other active or inactive ingredients besides listed above.
- Active Ingredient(s)
- Purpose
- Use
- Warnings
- Do not use
-
WHEN USING
When using this product keep out of eyes, ears, and mouth. In case of contact with eyes, rinse eyes thoroughly with water.
Stop use and ask a doctor if irritation or rash occurs. These may be signs of a serious condition.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away. - STOP USE
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other information
-
Inactive ingredients
water (aqua), glycerin (glycerol), Carbomer Copolymer Type A (Allyl Pentaerythritol Crosslinked), propylene glycol, Fragrance (Dimethyl Anthranilate), Triethanolamine, Dexpanthenol (Panthenol), Aloe Barbadensis Leaf Juice Powder, Beads [(Mannitol, Microcrystalline Cellulose, Hydroxypropyl Methylcellulose, Pigment Green 7 (CI 74260)], Blue 1 (CI 42090)
- Package Label - Principal Display Panel
-
INGREDIENTS AND APPEARANCE
HAND SANITIZER
ethyl alcohol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:75360-1125 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 80 mL in 100 mL Inactive Ingredients Ingredient Name Strength FD&C BLUE NO. 1 (UNII: H3R47K3TBD) 0.005 mL in 100 mL TROLAMINE (UNII: 9O3K93S3TK) 0.15 mL in 100 mL ALOE VERA LEAF (UNII: ZY81Z83H0X) 0.05 mL in 100 mL DIMETHYL ANTHRANILATE (UNII: 5Z37T562P9) 0.5 mL in 100 mL MANNITOL (UNII: 3OWL53L36A) HYPROMELLOSES (UNII: 3NXW29V3WO) PIGMENT GREEN 7 (UNII: BPO9294G4W) DEXPANTHENOL (UNII: 1O6C93RI7Z) 0.1 mL in 100 mL CARBOMER COPOLYMER TYPE A (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 71DD5V995L) 1.5 mL in 100 mL PROPYLENE GLYCOL (UNII: 6DC9Q167V3) 1 mL in 100 mL MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) GLYCERIN (UNII: PDC6A3C0OX) 5 mL in 100 mL WATER (UNII: 059QF0KO0R) 11.68 mL in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75360-1125-1 50 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/01/2020 2 NDC:75360-1125-2 65 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/01/2020 3 NDC:75360-1125-4 250 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/01/2020 4 NDC:75360-1125-5 1000 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/01/2020 5 NDC:75360-1125-3 100 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/01/2020 6 NDC:75360-1125-6 500 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/01/2020 7 NDC:75360-1125-7 400 mL in 1 BAG; Type 0: Not a Combination Product 04/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 04/01/2020 Labeler - Hemani International (645581294) Registrant - Hemani International (645581294) Establishment Name Address ID/FEI Business Operations Hemani International 645581294 manufacture(75360-1125)