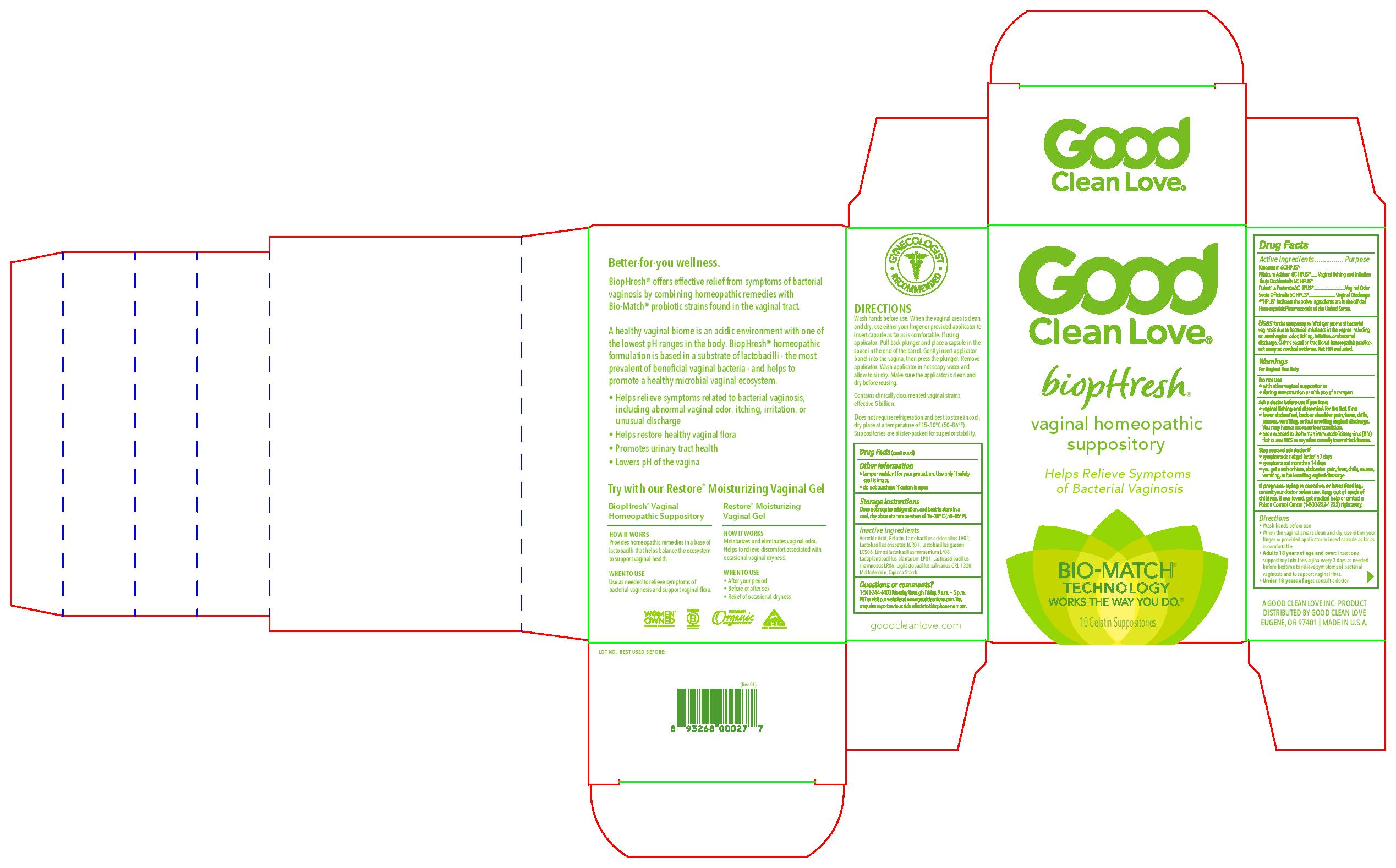
Label: BIOPHRESH- kreosotum 6c, nitricum acidum 6c, thuja occidentalis 6c, pulsatilla vulgaris 6c, sepia officinalis 6c suppository
- NDC Code(s): 73716-001-10
- Packager: Good Clean Love, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated December 27, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
-
PURPOSE
Kreosotum 6C HPUS*
Nitricum Acidum 6C HPUS*................Vaginal Itching and Irritation
Thuja Occidentalis 6C HPUS*
Pulsatilla Pratensis 6C HPUS*................Vaginal Odor
Sepia Officinalis 6C HPUS*................Vaginal Discharge
*"HPUS" indicates the active ingredients are in the official Homeopathic Pharmacopeia of the United States.
- INDICATIONS & USAGE
-
WARNINGS
For Vaginal Use Only
Ask a doctor before use if you have
- vaginal itching and discomfort for the first time
- lower abdominal, back or shoulder pain, fever, chills, nausea, vomiting, or foul smelling vaginal discharge. You may have a more serious condition.
- been exposed to the human immunodeficiency virus (HIV) that causes AIDS or any other sexually transmitted disease.
-
DOSAGE & ADMINISTRATION
Directions
- Wash hands before use
- When the vaginal area is clean and dry, use either your finger or providede applicator to insert capsule as far as is comfortable
- Adults 18 years of age and over: insert one suppository into the vagina every 3 days as needed before bedtime to relieve symptoms of bacterial vaginosis and to support vaginal flora.
- Under 18 years of age: consult a doctor
- OTHER SAFETY INFORMATION
- STORAGE AND HANDLING
-
INACTIVE INGREDIENT
Inactive ingredients
Ascorbic Acid, Gelatin, Lactobacillus acidophilus LA02, Lactobacillus crispatus LCR01, Lactobacillus gasseri LGS06, Limosilactobacillus fermentum LF08, Lactiplantibacillus plantarum LP01, Lacticaseibacillus rhamnosus LR06, Ligilactobacillus salivarius CRL1328, Maltodextrin, Tapioca starch
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BIOPHRESH
kreosotum 6c, nitricum acidum 6c, thuja occidentalis 6c, pulsatilla vulgaris 6c, sepia officinalis 6c suppositoryProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73716-001 Route of Administration VAGINAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength THUJA OCCIDENTALIS LEAFY TWIG (UNII: 1NT28V9397) (THUJA OCCIDENTALIS LEAFY TWIG - UNII:1NT28V9397) THUJA OCCIDENTALIS LEAFY TWIG 6 [hp_C] in 1 g PULSATILLA VULGARIS (UNII: I76KB35JEV) (PULSATILLA VULGARIS - UNII:I76KB35JEV) PULSATILLA VULGARIS 6 [hp_C] in 1 g SEPIA OFFICINALIS WHOLE (UNII: 48GD5780QF) (SEPIA OFFICINALIS WHOLE - UNII:48GD5780QF) SEPIA OFFICINALIS WHOLE 6 [hp_C] in 1 g WOOD CREOSOTE (UNII: 3JYG22FD73) (WOOD CREOSOTE - UNII:3JYG22FD73) WOOD CREOSOTE 6 [hp_C] in 1 g NITRIC ACID (UNII: 411VRN1TV4) (NITRIC ACID - UNII:411VRN1TV4) NITRIC ACID 6 [hp_C] in 1 g Inactive Ingredients Ingredient Name Strength STARCH, TAPIOCA (UNII: 24SC3U704I) LACTOBACILLUS PLANTARUM (UNII: QFC21096ON) LACTOBACILLUS RHAMNOSUS (UNII: 9601IVB87J) MALTODEXTRIN (UNII: 7CVR7L4A2D) LACTOBACILLUS GASSERI (UNII: Q66E0D2443) LACTOBACILLUS REUTERI (UNII: 9913I24QEE) LACTOBACILLUS SALIVARIUS (UNII: 7A4S6N2EG2) ASCORBIC ACID (UNII: PQ6CK8PD0R) GELATIN (UNII: 2G86QN327L) LACTOBACILLUS ACIDOPHILUS (UNII: 1PRR1V42V5) LACTOBACILLUS CRISPATUS (UNII: QX2H2M2084) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73716-001-10 1 g in 1 BLISTER PACK; Type 0: Not a Combination Product 04/17/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 04/17/2020 Labeler - Good Clean Love, Inc. (153909531) Establishment Name Address ID/FEI Business Operations Bonafide Innovations LLC 016579579 manufacture(73716-001)