Label: FUNGI NAIL TOE AND FOOT- tolnaftate solution
- NDC Code(s): 55505-177-26, 55505-177-80
- Packager: Kramer Laboratories
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 11, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- INDICATIONS & USAGE
- Warnings
- DO NOT USE
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions:
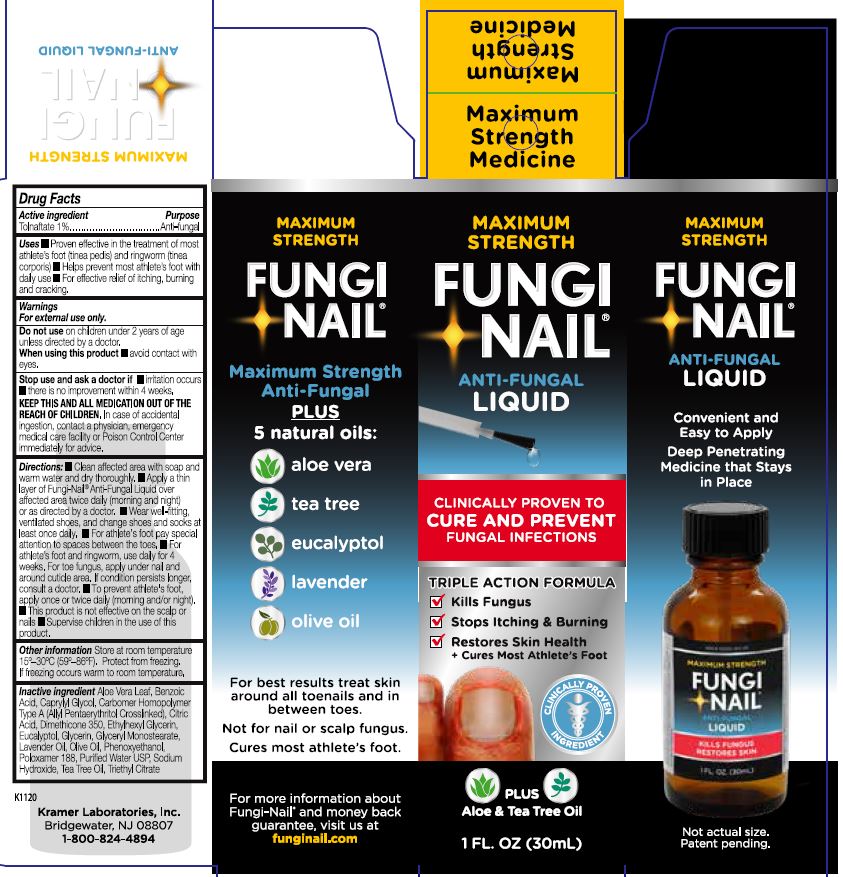
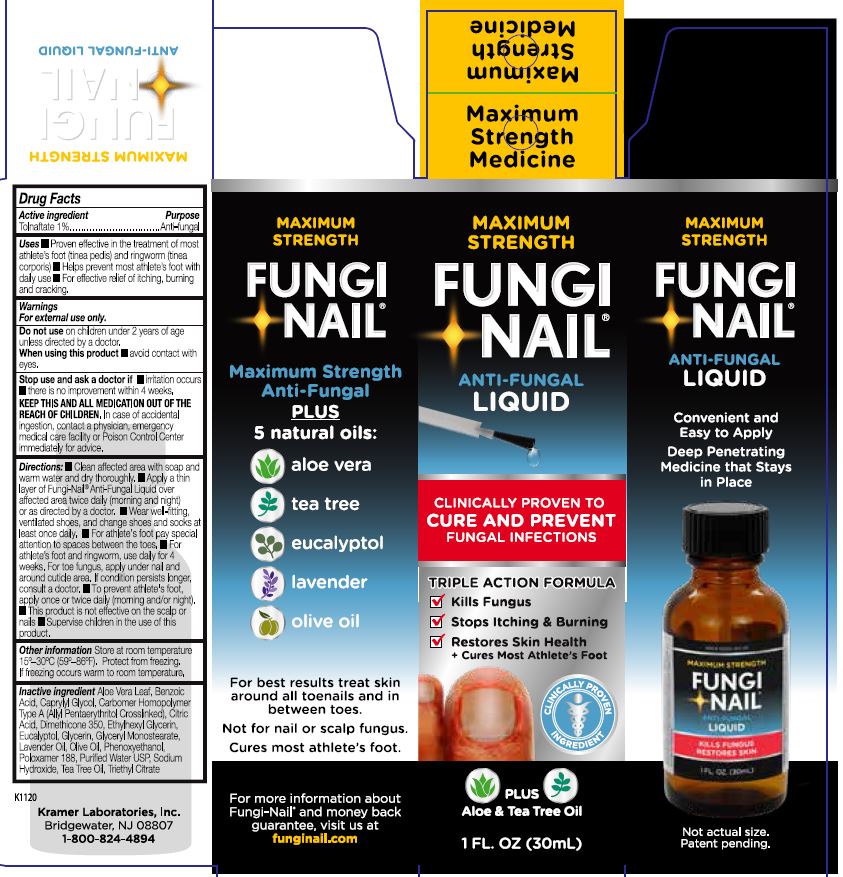
■ Clean affected areas with soap and warm water and dry thoroughly. ■ Apply a thin layer of Fungi-Nail® Anti-Fungal Liquid over affected area twice daily (morning and night) or as directed by a doctor. ■ Wear well-fitting, ventilated shoes, and change shoes and socks at least once daily. ■ For athlete’s foot pay special attention to spaces between the toes. ■ For athlete’s foot and ringworm, use daily for 4 weeks. For toe fungus, apply under nail and around cuticle area. If condition persists longer, consult a doctor. ■ To prevent athlete's foot, apply once or twice daily (morning and/or night). ■ This product is not effective on the scalp or nails. ■ Supervise children in the use of this product. - STORAGE AND HANDLING
-
INACTIVE INGREDIENT
Inactive ingredient
Aloe Vera Leaf, Benzoic Acid, Caprylyl Glycol, Carbomer Homopolymer Type A (Allyl Pentaerythritol Crosslinked), Citric Acid, Dimethicone 350, Ethylhexyl Glycerin, Eucalyptol, Glycerin, Glyceryl Monostearate, Lavender Oil, Olive Oil, Phenoxyethanol, Poloxamer 188, Purified Water USP, Sodium Hydroxide, Tea Tree Oil, Triethyl Citrate -
PRINCIPAL DISPLAY PANEL
MAXIMUM STRENGTH
FUNGI-NAIL®
ANTI-FUNGAL
LIQUID
CLINICALLY PROVEN TO
CURE AND PREVENT
FUNGAL INFECTIONS
TRIPLE ACTION FORMULA
✓ Kills Fungus
✓ Stops Itching & Burning
✓ Restores Skin Health
* Cures Most Athlete's Foot
CLINICALLY PROVEN INGREDIENT
PLUS
Aloe & Tea Tree Oil
1 FL. OZ. (30mL)
Convenient and Easy to Apply
Deep Penetrating Medicine that Stays in Place
Not actual size.
Patent pending.
Maximum Strength Anti-Fungal
PLUS
5 natural oils:
○ aloe vera
○ tea tree
○ eucalyptol
○ lavender
○ olive oil
For best results treat skin around all toenails and in between toes.
Not for nail or scalp fungus.
Cures most athlete's foot.
For more information about Fungi-Nail® and money back guarantee, visit us at funginail.com
Kramer Laboratories, Inc.
Bridgewater, NJ 08807
1-800-824-4894
K1120
MAXIMUM STRENGTH
ANTI-FUNGAL LIQUID
Maximum
Strength
Medicine
MAXIMUM STRENGTH
FUNGI-NAIL®
ANTI-FUNGAL
SPRAY
CLINICALLY PROVEN TO
CURE AND PREVENT
FUNGAL INFECTIONS
TRIPLE ACTION FORMULA
✓ Kills Fungus
✓ Stops Itching & Burning
✓ Restores Skin Health
+ Cures Most Athlete's Foot
CLINICALLY PROVEN INGREDIENT
PLUS
Aloe & Tea Tree Oil
1 FL. OZ. (30mL)
MAXIMUM
STRENGTH
FUNGI-NAIL®
ANTI-FUNGAL
SPRAY
Spray Applicator
Makes Application
Easy With No Mess
Deep Penetrating
Medicine that Stays
in Place
MAXIMUM
STRENGTH
FUNGI-NAIL®
ANTI-FUNGAL
SPRAY
Infused with
5 natural oils
○ aloe vera
○ tea tree
○ eucalyptol
○ lavender
○ olive oil
For best results treat skin
around all toenails and in
between toes.
Not for nail or scalp fungus.
Cures most athlete's foot.
For more information
about Fungi-Nail® and
money back guarantee,
visit us at funginail.com
Kramer Laboratories, Inc. Bridgewater, NJ 08807
1-800-824-4894
K0622

MAXIMUM STRENGTH
FUNGI-NAIL®
ANTI-FUNGAL
SPRAY
CLINICALLY PROVEN TO
CURE AND PREVENT
FUNGAL INFECTIONS
1 FL OZ (30mL)
Distributed by:
KRAMER
LABORITORIES
K0622
Kramer Laboratories, Inc. Bridgewater, NJ 08807
kramerlabs.com funginal.com 1-800-824-4894

-
INGREDIENTS AND APPEARANCE
FUNGI NAIL TOE AND FOOT
tolnaftate solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:55505-177 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Tolnaftate (UNII: 06KB629TKV) (Tolnaftate - UNII:06KB629TKV) Tolnaftate 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength Aloe Vera Leaf (UNII: ZY81Z83H0X) Benzoic Acid (UNII: 8SKN0B0MIM) Carbomer Homopolymer Type A (Allyl Pentaerythritol Crosslinked) (UNII: F68VH75CJC) Citric Acid Monohydrate (UNII: 2968PHW8QP) Dimethicone 350 (UNII: 2Y53S6ATLU) Ethylhexylglycerin (UNII: 147D247K3P) Eucalyptol (UNII: RV6J6604TK) Glycerin (UNII: PDC6A3C0OX) Glyceryl Monostearate (UNII: 230OU9XXE4) Lavender Oil (UNII: ZBP1YXW0H8) Olive Oil (UNII: 6UYK2W1W1E) Phenoxyethanol (UNII: HIE492ZZ3T) Poloxamer 188 (UNII: LQA7B6G8JG) Water (UNII: 059QF0KO0R) Sodium Hydroxide (UNII: 55X04QC32I) Tea Tree Oil (UNII: VIF565UC2G) Triethyl Citrate (UNII: 8Z96QXD6UM) Caprylyl Glycol (UNII: 00YIU5438U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55505-177-26 1 in 1 CARTON 03/25/2019 1 30 mL in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product 2 NDC:55505-177-80 1 in 1 CARTON 12/10/2022 2 30 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M005 03/25/2019 Labeler - Kramer Laboratories (122720675)