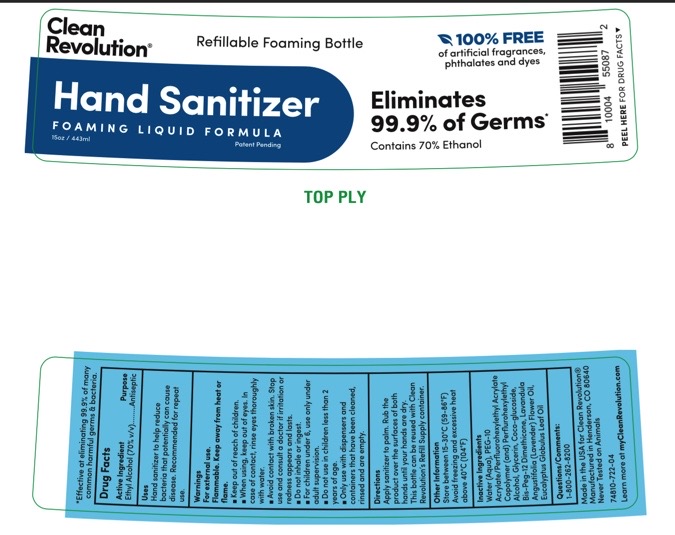
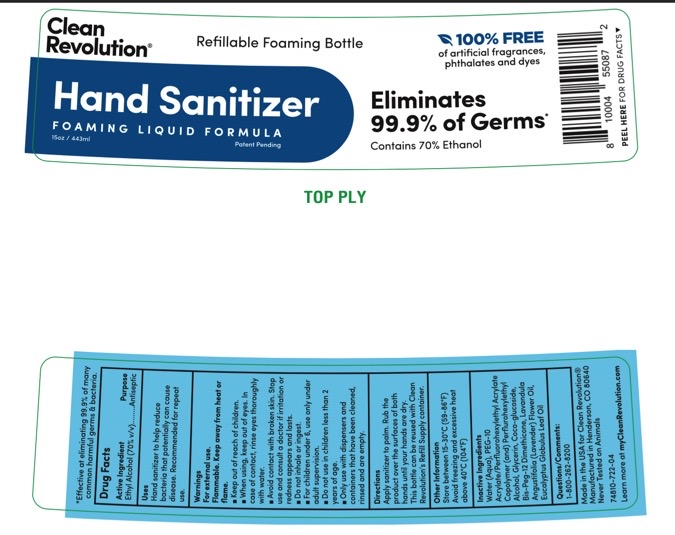
Label: CLEAN REVOLUTION- hand sanitizer liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 74810-722-03, 74810-722-04 - Packager: Replenish Bottling LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated July 1, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients.... Purpose
- Purpose
- Uses
- Warnings
- Warnings
- Warnings
- Warnings
- Warnings
- Warnings
- Warnings
-
Directions
1. Remove foil seal under cap.
2. For best results , pour into Clean Revolution® Refillable Foaming Bottle. To create foam, product must be dispensed from a foaming pump. Product can also be dispensed in a liquid consistency using a standard pump or spray bottle. Never mix this product with other household ingredients or pour into a bottle that previously contained other chemicals.
3. Apply sanitizer to palm. Rub the product over the surfaces of both hands until your hands are dry.
- Other Information
- Inactive Ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CLEAN REVOLUTION
hand sanitizer liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:74810-722 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 65.3 g in 100 g Inactive Ingredients Ingredient Name Strength LAVENDER OIL (UNII: ZBP1YXW0H8) 0.2 g in 100 g DECYL GLUCOSIDE (UNII: Z17H97EA6Y) 0.01 g in 100 g GLYCERIN (UNII: PDC6A3C0OX) 2 g in 100 g WATER (UNII: 059QF0KO0R) 28.289 g in 100 g BIS-PEG-12 DIMETHICONE (500 MPA.S) (UNII: 2CNS542YRT) 0.001 g in 100 g PEG-10 ACRYLATE/PERFLUOROHEXYLETHYL ACRYLATE COPOLYMER (UNII: D76Z87928N) 4 g in 100 g EUCALYPTUS OIL (UNII: 2R04ONI662) 0.2 g in 100 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:74810-722-03 100 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/07/2020 2 NDC:74810-722-04 100 g in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 07/07/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 07/07/2020 Labeler - Replenish Bottling LLC (044586187) Registrant - Replenish Bottling LLC (044586187)