Label: 62% ETHYL ALCOHOL ANTISEPTIC NASAL- ethyl alcohol swab
- NDC Code(s): 53329-132-29, 53329-132-78
- Packager: Medline Industries, LP
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated July 9, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Use
- Warnings
-
Directions
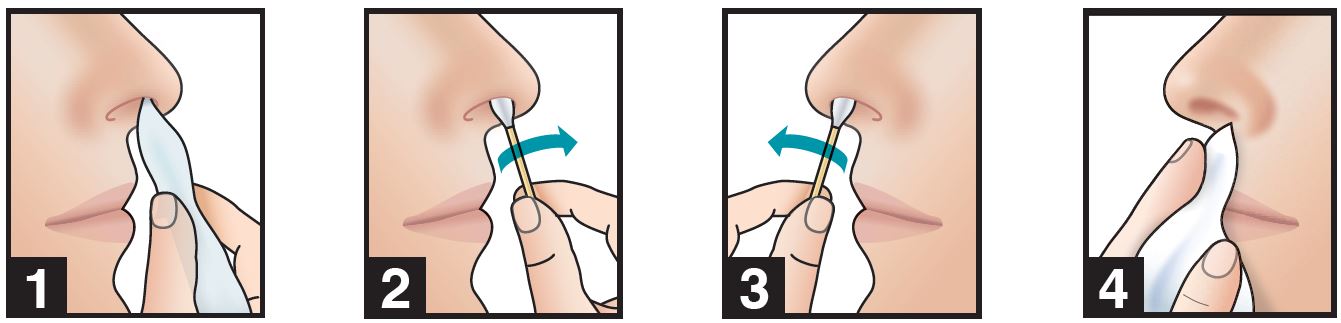
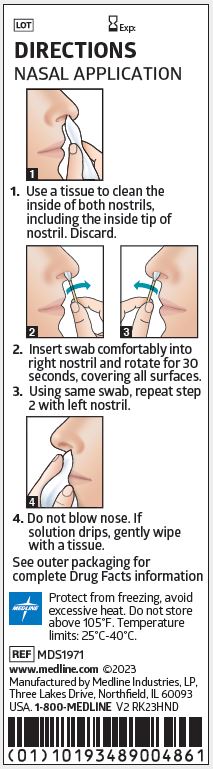
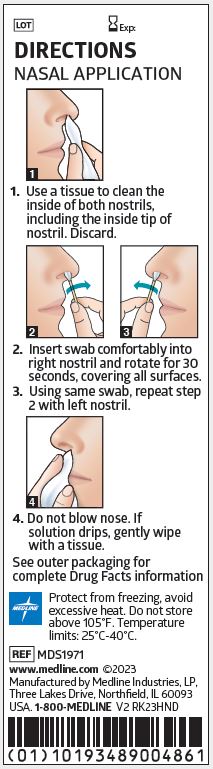
1. Use a tissue to clean the inside of both nostrils, including the inside tip of nostril. Discard.
2. Insert swab comfortably into right nostril and rotate for 30 seconds, covering all surfaces.
3. Using same swab, repeat step 2 with left nostril.
4. Do not blow nose. If solution drips, gently wipe with a tissue.
- Other information
- Inactive ingredients
- Manufacturing Information
- Package Label
-
INGREDIENTS AND APPEARANCE
62% ETHYL ALCOHOL ANTISEPTIC NASAL
ethyl alcohol swabProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:53329-132 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 62 mL in 100 mL Inactive Ingredients Ingredient Name Strength ASCORBYL PALMITATE (UNII: QN83US2B0N) CASTOR OIL (UNII: D5340Y2I9G) ISOPROPYL ALCOHOL (UNII: ND2M416302) BENZYL ALCOHOL (UNII: LKG8494WBH) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) MENTHOL (UNII: L7T10EIP3A) WATER (UNII: 059QF0KO0R) POLYAMINOPROPYL BIGUANIDE (UNII: DT9D8Z79ET) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:53329-132-29 50 in 1 BOX 05/01/2020 01/01/2025 1 NDC:53329-132-78 6 mL in 1 PACKET; Type 0: Not a Combination Product 2 NDC:53329-132-78 6 mL in 1 PACKET; Type 0: Not a Combination Product 05/01/2020 01/01/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 05/01/2020 01/01/2025 Labeler - Medline Industries, LP (025460908) Registrant - Medline Industries, LP (025460908)