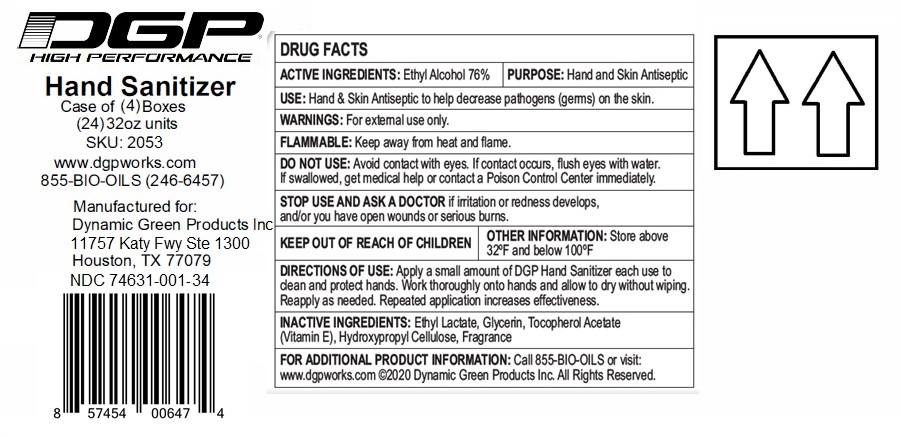
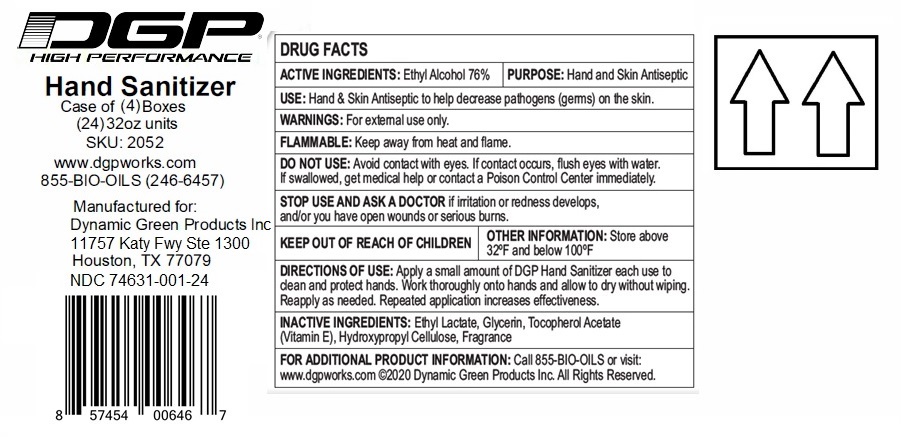
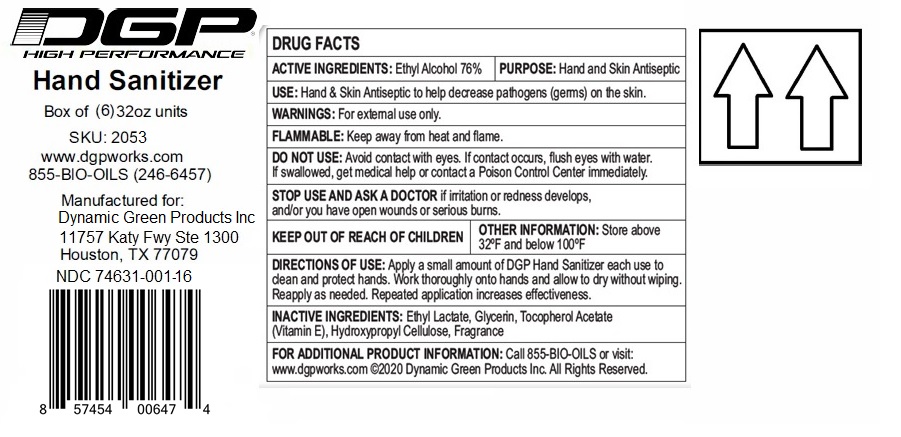
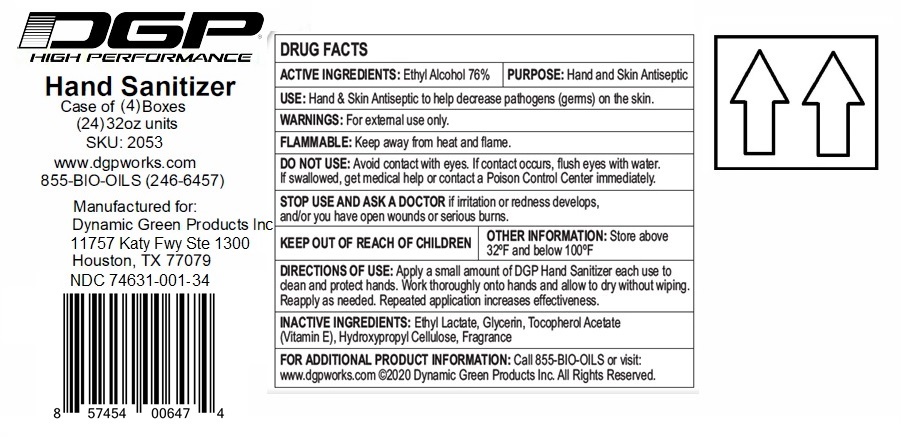
Label: DGP HIGH PERFORMANCE HAND SANITIZER- ethyl alcohol gel
-
Contains inactivated NDC Code(s)
NDC Code(s): 74631-001-01, 74631-001-02, 74631-001-06, 74631-001-16, view more74631-001-24, 74631-001-34 - Packager: Dynamic Green Products, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 16, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- INDICATIONS & USAGE
- WARNINGS
- Directions
- Inactive ingredients
-
Principal Display Panel
DGP(R) High Performance Hand Sanitizer
Bio-based Antibacterial Formula
Vitamin E+ Essential oils
To open pump, pinch safety lock and twist nozzle
To open refill, pull back tab to reveal pour hole
All ingredients are made in the USA
The packaging is sourced in the USA
The hand pump is non-domestic sourced.
(Manufactured for:)
Dynamic Green Products, Inc.
11757 Katy Fwy Ste 1300
Houston, TX 77079
For additional Product information: Call 855-BIO-OILS or visit: www.dgpworks.com
(C) Dynamic Green Products Inc. All Rights Reserved.
XX oz (xxx mL) NDC 74631-001-XX



-
INGREDIENTS AND APPEARANCE
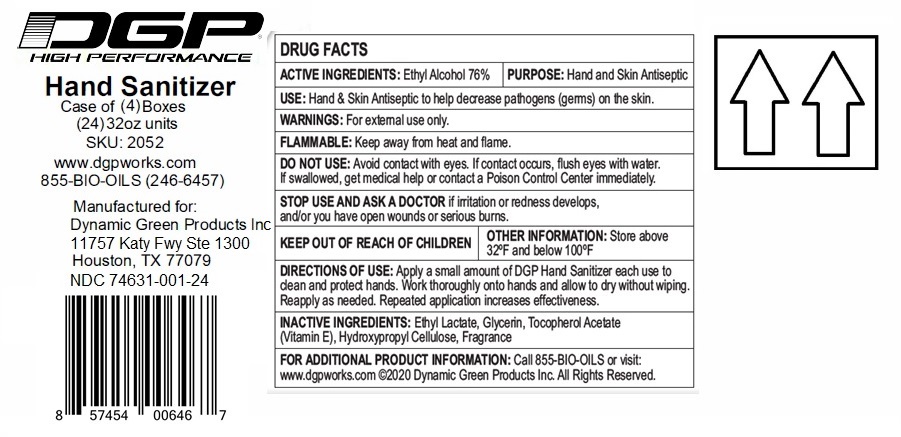
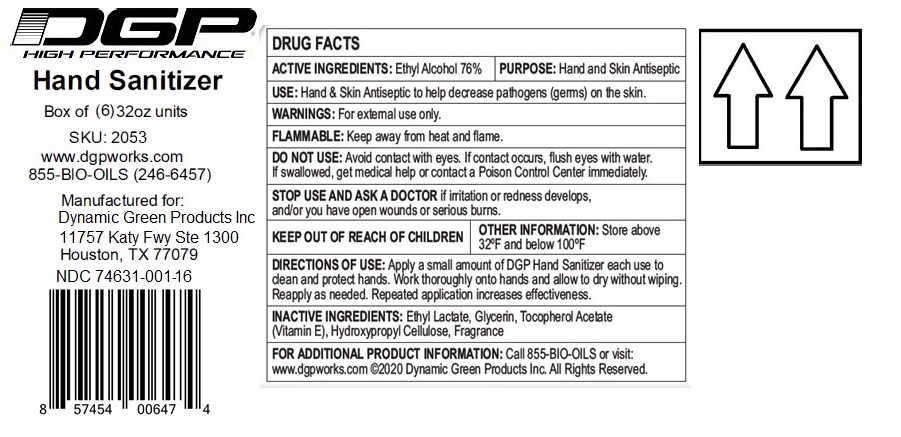
DGP HIGH PERFORMANCE HAND SANITIZER
ethyl alcohol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:74631-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 76 mL in 100 mL Inactive Ingredients Ingredient Name Strength HYDROXYPROPYL CELLULOSE (110000 WAMW) (UNII: 5Y0974F5PW) GLYCERIN (UNII: PDC6A3C0OX) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ETHYL LACTATE (UNII: F3P750VW8I) Product Characteristics Color Score Shape Size Flavor PEACH Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:74631-001-24 24 in 1 CASE 04/16/2020 1 NDC:74631-001-06 6 in 1 BOX 1 NDC:74631-001-01 946 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 2 NDC:74631-001-34 24 in 1 CASE 04/16/2020 2 NDC:74631-001-16 6 in 1 BOX 2 NDC:74631-001-02 946 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 04/16/2020 Labeler - Dynamic Green Products, Inc. (110949053) Establishment Name Address ID/FEI Business Operations Packaging Service Co 008435950 pack(74631-001) Establishment Name Address ID/FEI Business Operations Viking Chemical Company 025807272 manufacture(74631-001) , analysis(74631-001) Establishment Name Address ID/FEI Business Operations Dynamic Green Products Inc. 110949053 pack(74631-001) Establishment Name Address ID/FEI Business Operations Vertrauen Chemie Solutions Inc 080251121 label(74631-001)