Label: BITE AND STING RELIEF STICK (benzocaine, camphor- natural and phenol liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 69842-731-01 - Packager: CVS Health
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 13, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- PURPOSE
- Drug Facts
- Uses
-
Warnings
For external use only.
When using this product
■ Do not get in eyes ■ Do not apply over large areas of the body
■ In case of deep or puncture wounds, animal bites or serious
burns consult a doctor. ■ Do not bandage. -
Directions
■ Clean area ■ Apply a small amount to the bite area while massaging with sponge tip applicator. If itching persists, apply again in 10-15 minutes ■ For ticks and bees, remove tick or stinger before treatment ■ Adults and children 2 years and over: Apply to affected area 1 to 3 times daily ■ Children under 2 years: Consult a doctor
- Inactive ingredients
- Questions or Comments?
-
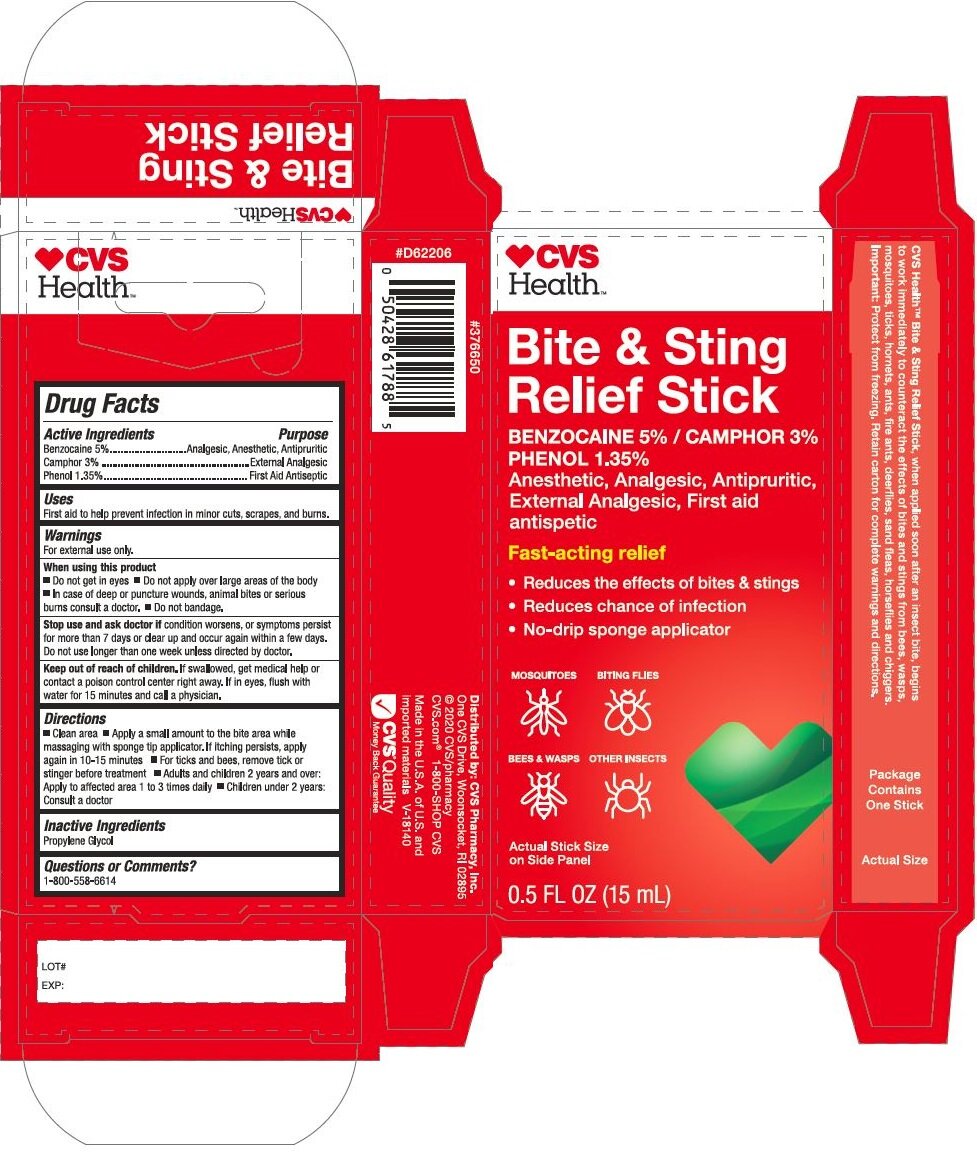
Principal Display Panel - 15 mL Stick Carton
CVS
HEALTH
Bite & Sting
Relief Stick
Benzocaine 5% / Phenol 1.35%
Camphor 3.0%
Anesthetic, Analgesic, Antipuritic,
External Analgesic, First Aid
antiseptic
Fast-acting relief
Reduces the effects of bites & stings
Reduces chance of infections
No-drip sponge applicatorMOSQUITOES BITING FLIES
BEES & WASPS OTHER INSECTS
Actual Stick Size
On Side Panel
0.5 FL. OZ. (15 mL)
- Principal Display Panel - 15 mL Tube Label
-
INGREDIENTS AND APPEARANCE
BITE AND STING RELIEF STICK
benzocaine, camphor (natural) and phenol liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69842-731 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 0.05 g in 1 g CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) (CAMPHOR (SYNTHETIC) - UNII:5TJD82A1ET) CAMPHOR (SYNTHETIC) 0.03 g in 1 g PHENOL (UNII: 339NCG44TV) (PHENOL - UNII:339NCG44TV) PHENOL 0.0135 g in 1 g Inactive Ingredients Ingredient Name Strength PROPYLENE GLYCOL (UNII: 6DC9Q167V3) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69842-731-01 1 in 1 CARTON 04/17/2020 1 14.79 g in 1 TUBE, WITH APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 04/17/2020 Labeler - CVS Health (062312574)