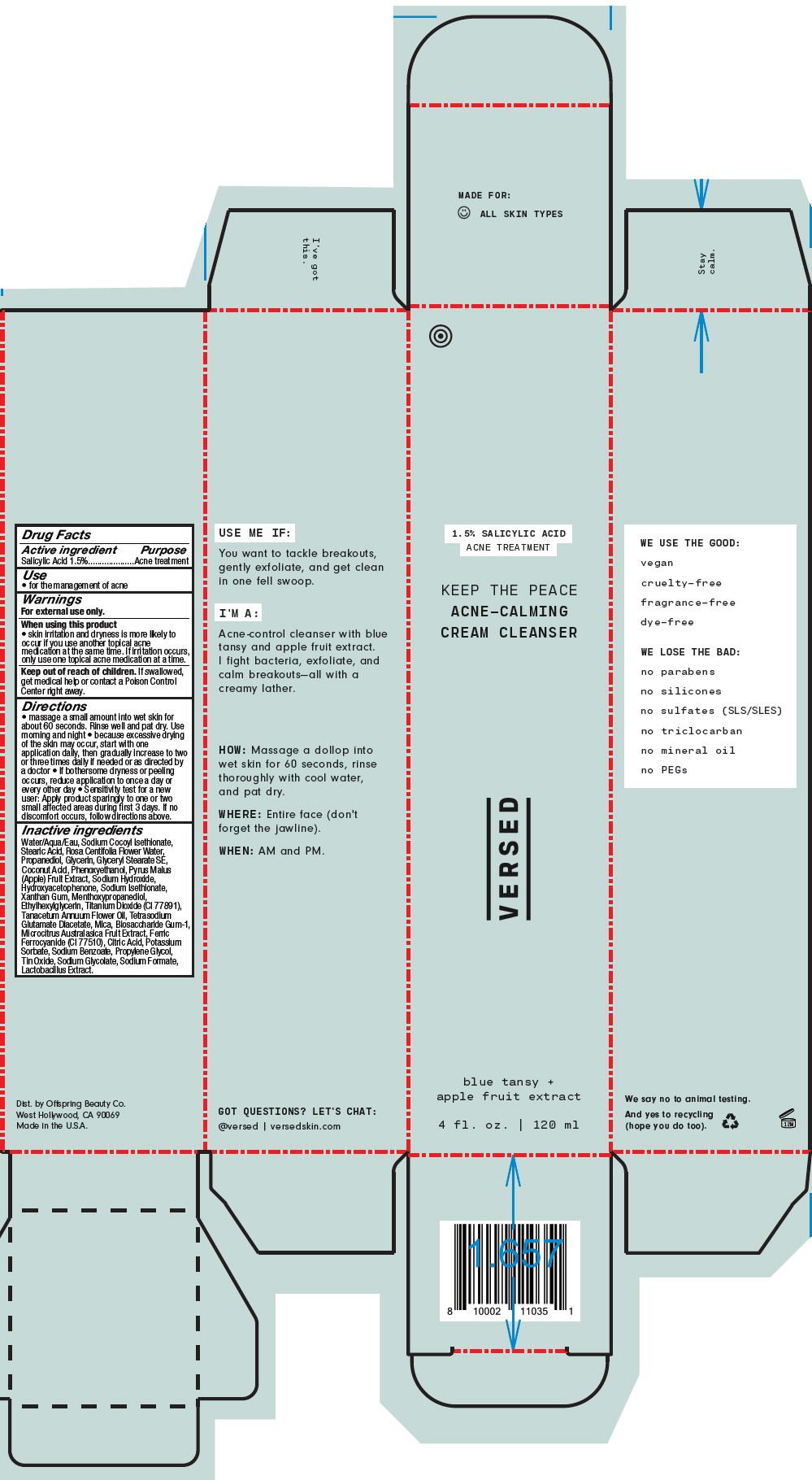
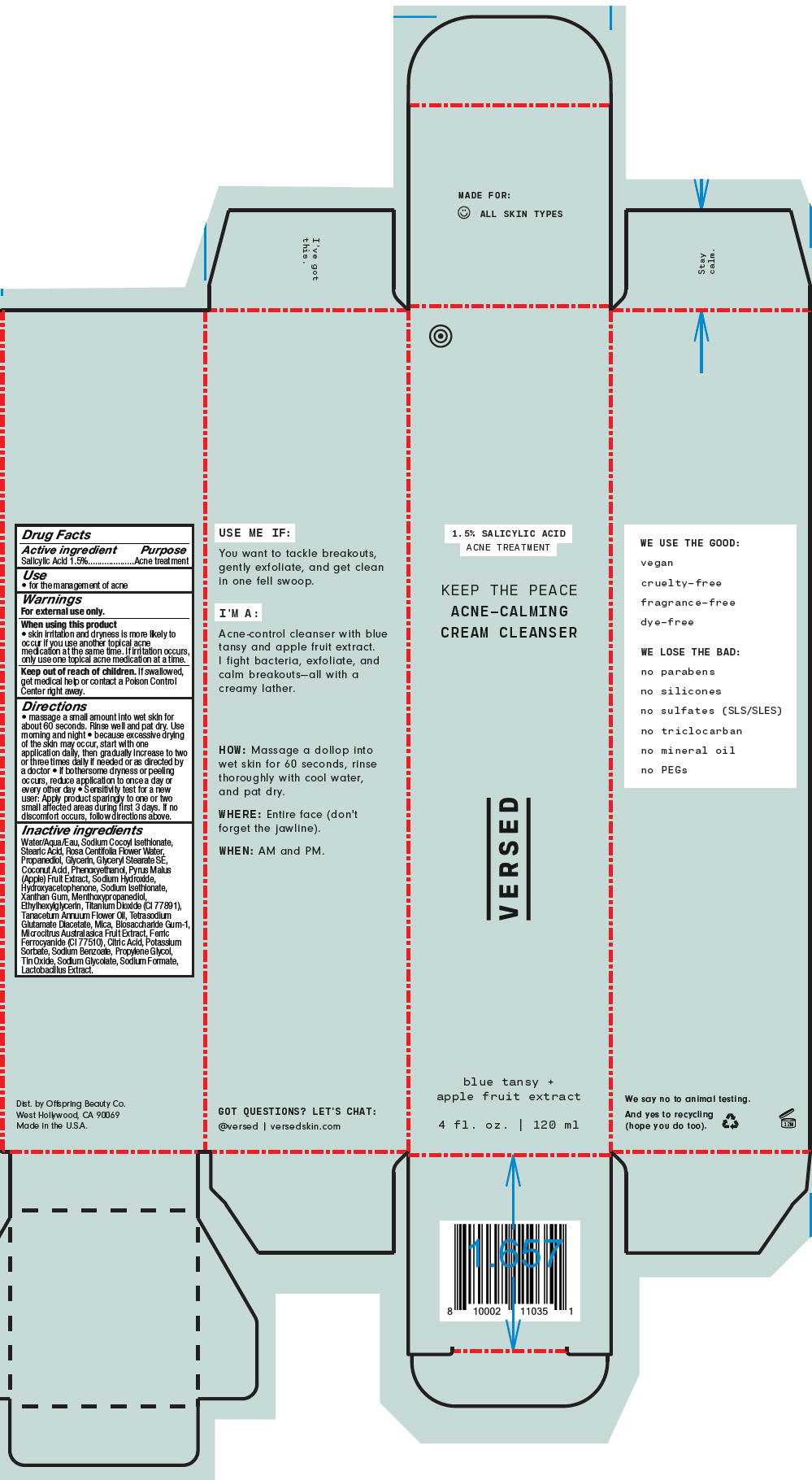
Label: KEEP THE PEACE ACNE-CALMING CREAM CLEANSER- salicylic acid rinse
- NDC Code(s): 73690-024-01
- Packager: Offspring Beauty Co. / Versed
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 9, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Use
- Warnings
-
Directions
- massage a small amount into wet skin for about 60 seconds. Rinse well and pat dry. Use morning and night
- because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor
- if bothersome dryness or peeling occurs, reduce application to once a day or every other day
- Sensitivity test for a new user: Apply product sparingly to one or two small affected areas during first 3 days. If no discomfort occurs, follow directions above.
-
Inactive ingredients
Water/Aqua/Eau, Sodium Cocoyl Isethionate, Stearic Acid, Rosa Centifolia Flower Water, Propanediol, Glycerin, Glyceryl Stearate SE, Coconut Acid, Phenoxyethanol, Pyrus Malus (Apple) Fruit Extract, Sodium Hydroxide, Hydroxyacetophenone, Sodium Isethionate, Xanthan Gum, Menthoxypropanediol, Ethylhexylglycerin, Titanium Dioxide (CI 77891), Tanacetum Annuum Flower Oil, Tetrasodium Glutamate Diacetate, Mica, Biosaccharide Gum-1, Microcitrus Australasica Fruit Extract, Ferric Ferrocyanide (CI 77510), Citric Acid, Potassium Sorbate, Sodium Benzoate, Propylene Glycol, Tin Oxide, Sodium Glycolate, Sodium Formate, Lactobacillus Extract.
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 120 ml Tube Carton
-
INGREDIENTS AND APPEARANCE
KEEP THE PEACE ACNE-CALMING CREAM CLEANSER
salicylic acid rinseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73690-024 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Salicylic Acid (UNII: O414PZ4LPZ) (Salicylic Acid - UNII:O414PZ4LPZ) Salicylic Acid 15 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SODIUM COCOYL ISETHIONATE (UNII: 518XTE8493) STEARIC ACID (UNII: 4ELV7Z65AP) ROSA CENTIFOLIA FLOWER OIL (UNII: H32V31VMWY) PROPANEDIOL (UNII: 5965N8W85T) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) PHENOXYETHANOL (UNII: HIE492ZZ3T) APPLE (UNII: B423VGH5S9) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) XANTHAN GUM (UNII: TTV12P4NEE) 3-((L-MENTHYL)OXY)PROPANE-1,2-DIOL (UNII: KD6TZ2QICH) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) TANACETUM ANNUUM FLOWERING TOP OIL (UNII: E2Q02N1ZC7) TETRASODIUM GLUTAMATE DIACETATE (UNII: 5EHL50I4MY) BIOSACCHARIDE GUM-1 (UNII: BB4PU4V09H) MICROCITRUS AUSTRALIS FRUIT (UNII: 9DNS80T428) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) LACTOBACILLUS BREVIS (UNII: 268IL53Q7O) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) MICA (UNII: V8A1AW0880) FERRIC FERROCYANIDE (UNII: TLE294X33A) COCONUT ACID (UNII: 40U37V505D) SODIUM ISETHIONATE (UNII: 3R36J71C17) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) SODIUM BENZOATE (UNII: OJ245FE5EU) STANNIC OXIDE (UNII: KM7N50LOS6) SODIUM GLYCOLATE (UNII: B75E535IMI) SODIUM FORMATE (UNII: 387AD98770) Product Characteristics Color BLUE (Sky Blue) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73690-024-01 1 in 1 CARTON 07/01/2020 1 120 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M006 07/01/2020 Labeler - Offspring Beauty Co. / Versed (081516405) Establishment Name Address ID/FEI Business Operations KDC/One Chatsworth, Inc. 118542196 MANUFACTURE(73690-024)