Label: COOLA BARE REPUBLIC MINERAL FACE-LOTION SPF 70- titanium dioxide 3.5% zinc oxide 15.8% lotion
- NDC Code(s): 79753-041-01
- Packager: COOLA LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 20, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
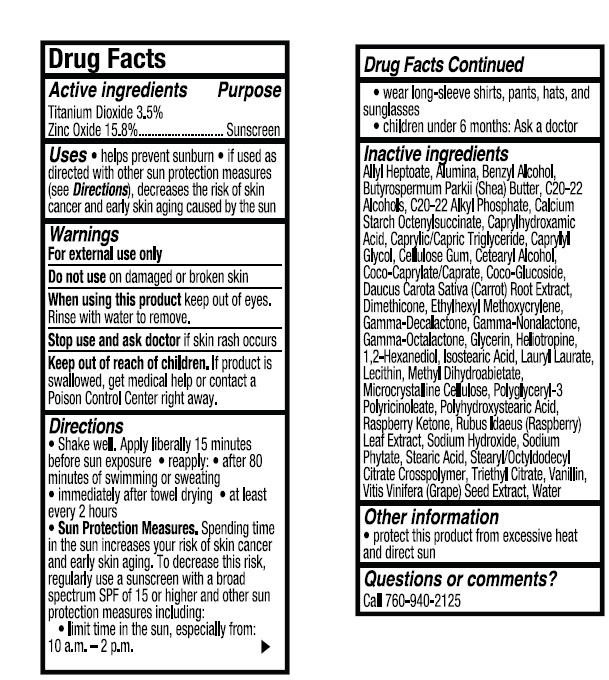
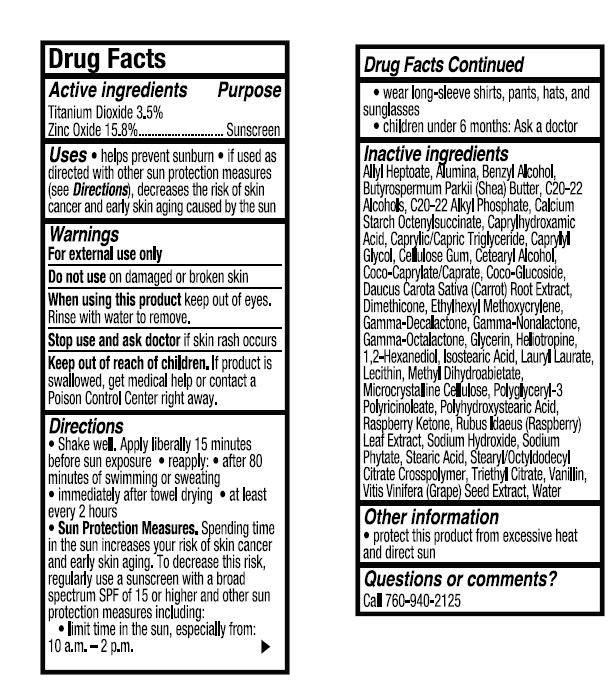
- ACTIVE INGREDIENT
- INDICATIONS & USAGE
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions
● Shake well. Apply liberally 15 minutes
before sun exposure ● reapply: ● after 80
minutes of swimming or sweating
● immediately after towel drying ● at least
every 2 hours
● Sun Protection Measures. Spending time
in the sun increases your risk of skin cancer
and early skin aging. To decrease this risk,
regularly use a sunscreen with a broad
spectrum SPF of 15 or higher and other sun
protection measures including:
● limit time in the sun, especially from:
10 a.m. - 2 p.m.
● wear long-sleeve shirts, pants, hats, and
sunglasses
●Children under 6 months: Ask a doctor -
INACTIVE INGREDIENT
Inactive Ingredients
Allyl Heptoate, Alumina, Benzyl Alcohol,
Butyrospermum Parkii (Shea) Butter, C20-22
Alcohols, C20-22 Alkyl Phosphate, Calcium
Starch Octenylsuccinate, Caprylhydroxamic
Acid, Caprylic/Capric Triglyceride, Caprylyl
Glycol, Cellulose Gum, Cetearyl Alcohol,
Coco-Caprylate/Caprate, Coco-Glucoside,
Daucus Carota Sativa (Carrot) Root Extract,
Dimethicone, Ethylhexyl Methoxycrylene,
Gamma-Decalactone, Gamma-Nonalactone,
Gamma-Octalactone, Glycerin, Heliotropine,
1,2-Hexanediol, Isostearic Acid, Lauryl Laurate,
Lecithin, Methyl Dihydroabietate,
Microcrystalline Cellulose, Polyglyceryl-3
Polyricinoleate, Polyhydroxystearic Acid,
Raspberry Ketone, Rubus Idaeus (Raspberry)
Leaf Extract, Sodium Hydroxide, Sodium
Phytate, Stearic Acid, Stearyl/Octyldodecyl
Citrate Crosspolymer, Triethyl Citrate, Vanillin,
Vitis Vinifera (Grape) Seed Extract, Water
- OTHER SAFETY INFORMATION
- SPL UNCLASSIFIED SECTION
-
COOLA Label
SPF
70
BARE
REPUBLIC
MINERAL
Sunscreen Lotion
Non-Nano Zinc Oxide
Face
Sheer, Oil-Free Finish
Broad Spectrum SPF 70
Water Resistant (80 Minutes)
2.0 FL OZ / 59 mL
*Hawaii Reef Friendly Sunscreen Oxybenzone & Octinoxate Free
©2021 COOLA. Distributed by: COOLA LLC, located in Sunny & CoolSan Diego, CA 92010.

res
-
INGREDIENTS AND APPEARANCE
COOLA BARE REPUBLIC MINERAL FACE-LOTION SPF 70
titanium dioxide 3.5% zinc oxide 15.8% lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:79753-041 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 15.8 g in 100 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 3.5 g in 100 mL Inactive Ingredients Ingredient Name Strength ALLYL HEPTANOATE (UNII: AU4CYG9V68) BENZYL ALCOHOL (UNII: LKG8494WBH) BUTYROSPERMUM PARKII (SHEA) BUTTER UNSAPONIFIABLES (UNII: 0C9AC7D6XU) C20-22 ALCOHOLS (UNII: O4M0347C6A) C20-22 ALKYL PHOSPHATE (UNII: L4VKP0Y7RP) CAPRYLHYDROXAMIC ACID (UNII: UPY805K99W) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) COCO GLUCOSIDE (UNII: ICS790225B) CARROT (UNII: L56Z1JK48B) DIMETHICONE (UNII: 92RU3N3Y1O) ETHYLHEXYL METHOXYCRYLENE (UNII: S3KFG6Q5X8) .GAMMA.-DECALACTONE (UNII: 7HLS05KP9O) .GAMMA.-NONALACTONE (UNII: I1XGH66S8P) .GAMMA.-OCTALACTONE (UNII: UHD6M52X0K) GLYCERIN (UNII: PDC6A3C0OX) PIPERONAL (UNII: KE109YAK00) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) ISOSTEARIC ACID (UNII: X33R8U0062) LAURYL LAURATE (UNII: GPW77G0937) LECITHIN, SUNFLOWER (UNII: 834K0WOS5G) METHYL DIHYDROABIETATE (UNII: 7666FJ0J9F) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYGLYCERYL-3 PENTARICINOLEATE (UNII: 7Q0OK5DOT4) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) 4-(P-HYDROXYPHENYL)-2-BUTANONE (UNII: 7QY1MH15BG) RUBUS IDAEUS LEAF (UNII: 8O2V33JG64) SODIUM HYDROXIDE (UNII: 55X04QC32I) DISODIUM PHTHALATE (UNII: QB14YV193C) STEARIC ACID (UNII: 4ELV7Z65AP) STEARYL/OCTYLDODECYL CITRATE CROSSPOLYMER (UNII: PN88NW0KPK) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) VANILLIN (UNII: CHI530446X) VITIS VINIFERA SEED (UNII: C34U15ICXA) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:79753-041-01 59 mL in 1 TUBE; Type 0: Not a Combination Product 05/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 05/01/2020 Labeler - COOLA LLC (956990290)