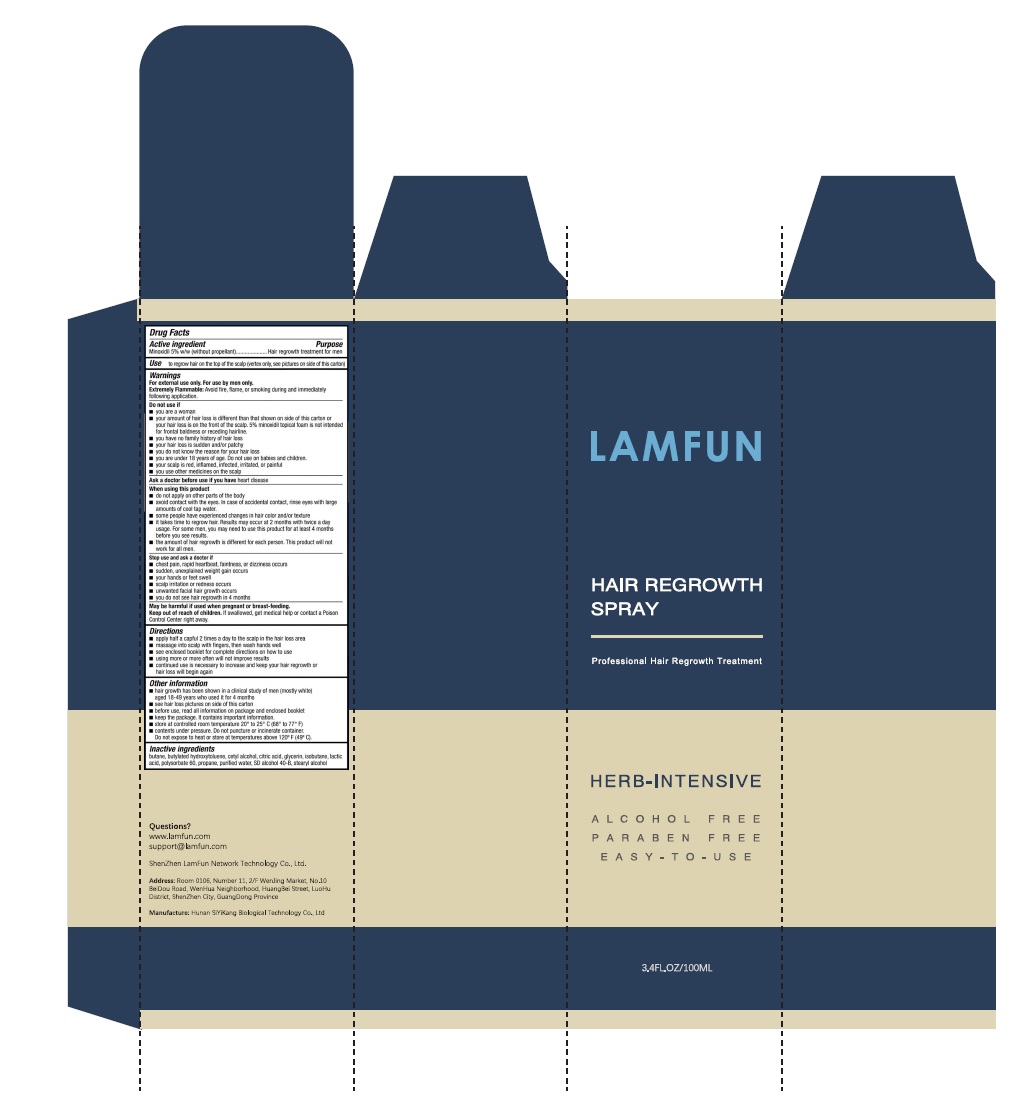
Label: LAMFUN HAIR REGROWTH TREATMENT- minoxidil solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 74770-001-01 - Packager: Shenzhen Lanfan Network Technology Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 6, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DOSAGE & ADMINISTRATION
apply half a capful 2 times a day to the scalp in the hair loss area
massage into scalp with fingers, then wash hands well
see enclosed booklet for complete directions on how to use
using more or more often will not improve results
continued use is necessary to increase and keep your hair regrowth or loss will begin again
- WARNINGS
- INDICATIONS & USAGE
- KEEP OUT OF REACH OF CHILDREN
- PURPOSE
- ACTIVE INGREDIENT
- INACTIVE INGREDIENT
- INACTIVE INGREDIENT
- INACTIVE INGREDIENT
- Package Label - Principal Display Panel
-
INGREDIENTS AND APPEARANCE
LAMFUN HAIR REGROWTH TREATMENT
minoxidil solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:74770-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MINOXIDIL (UNII: 5965120SH1) (MINOXIDIL - UNII:5965120SH1) MINOXIDIL 5 g in 100 mL Inactive Ingredients Ingredient Name Strength BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) BUTANE (UNII: 6LV4FOR43R) CETYL ALCOHOL (UNII: 936JST6JCN) GLYCERIN (UNII: PDC6A3C0OX) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:74770-001-01 100 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/06/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076239 04/06/2020 Labeler - Shenzhen Lanfan Network Technology Co., Ltd. (417999420) Establishment Name Address ID/FEI Business Operations Shenzhen Lanfan Network Technology Co., Ltd. 417999420 manufacture(74770-001)